5-hydroxytryptophan
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| L -5-hydroxytryptophan | ||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Non-proprietary name | Oxitriptan | |||||||||||||||||||||
| other names | ||||||||||||||||||||||
| Molecular formula | C 11 H 12 N 2 O 3 | |||||||||||||||||||||
| Brief description |
whitish powder, finely crystalline |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Drug information | ||||||||||||||||||||||
| ATC code | ||||||||||||||||||||||
| Drug class | ||||||||||||||||||||||
| Mechanism of action |
Hormone precursor |
|||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 220.23 g · mol -1 | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| density | ||||||||||||||||||||||
| Melting point |
270 ° C |
|||||||||||||||||||||
| solubility |
poorly soluble in water |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
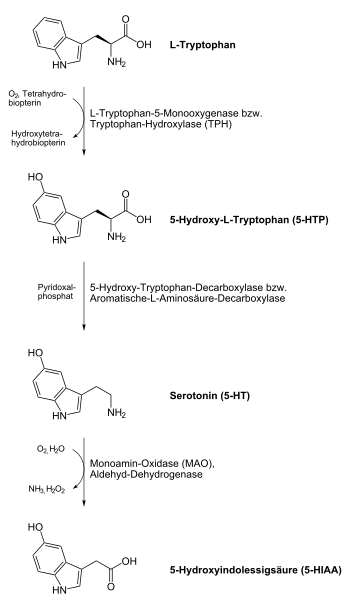
5-Hydroxytryptophan ( 5-HTP ), more precisely L -5-Hydroxytryptophan [Synonym: ( S ) -5-Hydroxytryptophan], non- proprietary name : Oxitriptan , is a non- proteinogenic α- amino acid with a lipophilic aromatic side chain. It is an intermediate product in the synthesis of serotonin from L- tryptophan in organisms.
Occurrence and extraction
Like L - tryptophan and serotonin, L -5-hydroxytryptophan occurs in various types of bananas . Commercially it is obtained from the seeds of the African black bean ( Griffonia simplicifolia ), in which it is abundant.
Biological importance in humans
metabolism
L -5-Hydroxytryptophan has no biological function of its own in the human body, but is used in the human body for the synthesis of serotonin (and consequently also for the synthesis of melatonin). In contrast to its chemical precursor L - tryptophan, it has no other noteworthy metabolic pathways, i.e. it is completely converted into serotonin . In addition, the conversion of L -5-HTP to serotonin is much faster than that of L- tryptophan to 5-HTP. For these two reasons, the effect of L -5-HTP on the serotonin balance is faster and stronger than that of L- tryptophan.
Most of the L -5-HTP ingested through food is metabolized in the liver and released into the blood as serotonin , where it is partly absorbed by platelets , partly by intestinal cells, and partly quickly broken down again by the monoamino-oxidase system of the lungs becomes. Another part passes through the blood-brain barrier into the brain , where it is of the serotonergic neurons used for serotonin synthesis.
The particular cell type of enterochromaffin cells located in the intestine should be mentioned here in particular , which represent an intermediate form between nerve cells and simple intestinal cells and also use serotonin for signal transmission. Since these cells have the enzyme hydroxytryptophan decarboxylase , but not tryptophan hydroxylase , they cannot normally produce serotonin from food themselves; because our food usually only contains L- Triptophan and no (or hardly any) L -5-HTP. They are therefore dependent on the L -5-HTP, which has been produced in the liver from L- tryptophan and has not yet been converted into serotonin. If L -5-HTP is now taken directly with food or as a food supplement , the amount of L -5-HTP that reaches these cells is usually much larger than usual and they start to produce a lot of serotonin from it. The result are some of the side effects of L -5-HTP that L- Triptophan does not have, namely - depending on the dose - loss of appetite , nausea and vomiting .
5-HTP and serotonin biosynthesis
L - Tryptophan is converted into L -5-hydroxytryptophan ( L -5-HTP) by the enzyme tryptophan hydroxylase (TPH) . Since the transition from L- tryptophan to L -5-HTP is the rate-determining step in the body's own serotonin synthesis, tryptophan hydroxylase plays an important regulating role in this synthesis pathway.
L -5-HTP is converted into serotonin by the enzyme hydroxytryptophan decarboxylase (more precisely: aromatic L-amino acid decarboxylase , AADC) . The vitamin B 6 derivative pyridoxal phosphate acts as a cofactor and, depending on its presence, increases or decreases the activity of hydroxytryptophan decarboxylase.
pharmacology
For L -5-hydroxytryptophan (as well as from L - tryptophan ), a mood, soothing and weight loss effects adopted. For example, a study on rats with L-5-HTP, which occurs naturally in the African black bean, showed a reduction in fear of open spaces, from which the researchers deduced a potential use in humans as an anxiolytic . The effect is based on the metabolism to serotonin in the human body. It is believed that increased levels of serotonin can improve mood and relieve depression . Regardless of this, the clinical efficacy of L -5-hydroxytryptophan is considered insufficiently proven due to older and qualitatively inadequate study data. In a small study on 20 overweight people, L-5-HTP after oral administration as a spray increased the feeling of satiety while at the same time reducing the body mass index (BMI).
In order to minimize the peripheral serotonin effects, which are undesirable effects of systemic L -5-hydroxytryptophan administration, L -5-hydroxytryptophan is often used together with a "peripheral" decarboxylase inhibitor (PDI). These drugs, which include carbidopa , for example , inhibit the metabolism of L -5-hydroxytryptophan to serotonin in the periphery, but not in the brain, as they do not cross the blood-brain barrier .
Pharmacokinetics
After oral administration, 50–85% of L -5-hydroxytryptophan is absorbed into the systemic circulation. Here it is about 60% bound to plasma proteins. It is almost completely metabolized to serotonin and excreted in the urine in the form of 5-hydroxyindolylacetic acid . Its plasma half-life is about 2 to 7 hours.
Side effects and interactions
In therapeutic use can be at higher doses side effects of gastrointestinal tract may occur, especially nausea, vomiting and diarrhea. Headaches , insomnia and palpitation are less common . The rate of peripheral side effects is lower when a peripheral decarboxylase inhibitor is administered at the same time .
In 1998, the FDA reported ten people worldwide who may have developed eosinophilia-myalgia syndrome (EMS) in connection with the use of L- 5-hydroxytryptophan supplements. However, indications of a causal relationship with the active ingredient could not be confirmed. Instead, contamination with tryptophan-4,5-dione in previous L -5- hydroxytryptophan preparations was believed to be the cause of the syndrome.
If L -5-hydroxytryptophan is used at the same time as other drugs with an effect on the serotonin system, such as selective serotonin reuptake inhibitors and at least theoretically MAO inhibitors and tricyclic antidepressants , there is a risk of a so-called serotonin syndrome . This is caused by uncontrollably high serotonin levels and is characterized, among other things, by high blood pressure , fever , flushing , dizziness, confusion and cramps.
Another side effect of 5-hydroxytryptophan is the reduced formation of dopamine and other catecholamines , as their biosynthesis partly requires the same chemical precursors as that of serotonin. The increased formation of serotonin at the expense of dopamine can occur, particularly with prolonged use of 5-HTP. If there is a connection between catecholamines and illness in the present depression illness, the long-term use of 5-HTP in particular could have a negative effect on the course of the illness.
use
Under the trade name Levothym , L -5-hydroxytryptophan was used as a prescription drug for the treatment of depression , particularly in the 1970s and 1980s . The market withdrawal in Germany took place in 1992. Since the introduction of the so-called selective serotonin reuptake inhibitors (SSRI) towards the end of the 1980s, L- 5-hydroxytryptophan has hardly had any therapeutic significance in this indication. In Germany, there is no approved drug with the active ingredient L -5-hydroxytryptophan on the market. Other areas of application are the treatment of post-anoxic myoclonus , the substitute treatment for a biopterin synthesis defect and the prophylaxis of migraines and headaches.
Griffonia extracts rich in 5-HTP are sold in Germany as dietary supplements . According to Löbell-Behrends et al. However, the seed extracts from Griffonia simplicifolia are products to be classified as novel foods (novel foods, novel food ingredients) that are subject to approval in the European Union. Whether Griffonia extracts are legally or may be marketed must be checked on a case-by-case basis and is the responsibility of the competent authorities.
No health-related advertising claims are permitted under the Health Claims Regulation .
Web links
- Alexander Römler: 5-Hydroxy-Tryptophan (5HTP). A helpful precursor to serotonin . (PDF; 481 kB) 2010.
Individual evidence
- ↑ a b c d e f Data sheet 5-Hydroxy-L-tryptophan - CAS 895096 - Calbiochem (PDF) from Merck , accessed on December 25, 2019.
- ↑ 5-Hydroxytryptophan data sheet at Acros, accessed December 25, 2019.
- ↑ a b c Entry on 5-hydroxytryptophan in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ MK Rahman, T. Nagatsu, T. Sakurai, S. Hori, M. Abe, M. Matsuda: Effect of pyridoxal phosphate deficiency on aromatic L-amino acid decarboxylase activity with L-DOPA and L-5-hydroxytryptophan as substrates in Board . In: Jpn J Pharmacol . tape 32 , no. 5 , October 1982, p. 803-811 , PMID 6983619 .
- ↑ Aromatic- L- amino acid decarboxylase (EC 4.1.1.28). In: Expasy.org
- ↑ G. Carnevale et al. a .: Anxiolytic-like effect of "Griffonia simplicifolia" Baill. seed extract in rats. In: Phytomedicine . 18, 2011, pp. 848-851. PMID 21353511 .
- ↑ K. Shaw, J. Turner, C. Del Mar: Tryptophan and 5-Hydroxytryptophan for depression. In: Cochrane Database of Systematic Reviews. Issue 1, 2002, Art. No .: CD003198. doi: 10.1002 / 14651858.CD003198
- ↑ M. Rondanelli, A. Opizzi, M. Faliva, M. Bucci, S. Perna: Relationship between the absorption of 5-hydroxytryptophan from diet to integrated, by Means of Griffonia simplicifolia extract, and the effect on satiety in overweight females after oral spray administration. In: Eat Weight Disord. 17 (1), 2012, pp. E22 – e28. doi: 10.3275 / 8165 . Epub 2011 Dec 5
- ↑ HJ Gijsman, JM van Gerven, ML u de Kam. a .: Placebo-controlled comparison of three dose regimens of 5-hydroxytryptophan challenge test in healthy volunteers . In: J Clin Psychopharmacol . tape 22 , no. 2 , April 2002, p. 183-189 , PMID 11910264 .
- ↑ Open Drug Database | Medication | Specialist information on Tript-OH®. ( Memento from December 5, 2009 in the Internet Archive )
- ^ HG Westenberg, TW Gerritsen, BA Meijer, HM van Praag: Kinetics of l-5-hydroxytryptophan in healthy subjects . In: Psychiatry Res . tape 7 , no. 3 , December 1982, pp. 373-385 , PMID 6187038 .
- ↑ WF Byerley, LL Judd, FW Reimherr, BI Grosser: 5-Hydroxytryptophan: a review of its antidepressant efficacy and adverse effects . In: J. Clin. Psychopharmacol . tape 7 , no. 3 , June 1987, pp. 127-137 , PMID 3298325 .
- ↑ a b c E. H. Turner, JM Loftis, AD Blackwell: Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan . In: Pharmacol Ther . tape 109 , no. 3 , March 2006, p. 325–338 , doi : 10.1016 / j.pharmthera.2005.06.004 , PMID 16023217 .
- ↑ M. Hinz, A. Stein, T. Uncini: 5-HTP efficacy and contraindications . In: Neuropsychiatr Dis Treat. tape 2012 , no. 8 , July 19, 2012, p. 323–328 , doi : 10.2147 / NDT.S33259 , PMID 22888252 , PMC 3415362 (free full text).
- ↑ Withdrawal of oxitriptan (LEVOTHYM). drug telegram 2/92.
- ↑ technical information Tript-OH , Sigma-Tau SpA (Italy), as of June 2016th
- ↑ Levotonine , Haute Autorité de Santé (France) March, 2014.
- ↑ a b Levothym, information in the AMIS of the German drug authorities, as of September 1992.
- ↑ Technical information Cincofarm , Angelini Farmacêutica, Lda. (Portugal) as of July 2007.
- ↑ S. Löbell-Behrends et al .: Borderline products - control of Internet trade with anti-aging and slimming products. A pilot study . ( Memento from January 6, 2018 in the Internet Archive ) (PDF) In: Deutsche Lebensmittel-Rundschau , 104th volume, issue 6, 2008, p. 268.
- ↑ EU Register on Nutrition and Health Claims. ec.europa.eu