Biopterin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
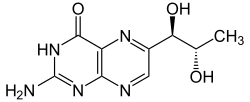
| Structural formula of L - erythro- biopterin | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Biopterin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 11 N 5 O 3 | |||||||||||||||
| Brief description |
pale yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 237.22 g mol −1 | |||||||||||||||
| Melting point |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Biopterin is a heterocyclic compound useful as redox - cofactor in the metabolism is important. The main structural feature is a heterocyclic pteridine ring system, making it a derivative of pterine .
Biochemically, biopterin is formed by the oxidation of tetrahydrobiopterin with GTP .
history
The compound was discovered by four groups of researchers in different sources in the 1950s. In the USA, a working group from Lederle Laboratories, a division of the American Cyanamid Company, isolated about 20 milligrams of a substance from 4000 liters of human urine by adsorption on activated carbon , countercurrent distribution and chromatography , which in a biological test promoted the growth of the protozoan Crithidia fasciculata . E. L. Patterson called her biopterin and derived the structural formula from degradation experiments.
Independently of this, HS Forrest and HK Mitchell from the California Institute of Technology (Pasadena) reported in the same year that they had isolated the substance, along with other pteridines, from the fruit fly Drosophila melanogaster (wild type). They made the same structural proposal.
At the Chemical Institute of the University of Zurich had Max Viscontini and employees (where the substance BH 2 called) also melanogaster Drosophila discovered.
Finally, at the Max Planck Institute in Munich, Adolf Butenandt and Heinz Rembold found biopterin in the queen cell feed juice ( royal jelly ) of the honey bee ( Apis mellifica ).
In the period that followed, biopterin was found in numerous organisms; its occurrence is ubiquitous, which is understandable from its biochemical function (see below).
properties
Biopterin forms small, yellow crystals with a spherical habit, which char when heated to 250 to 280 ° C without melting. They are sparingly soluble in water, but better both in dilute hydrochloric acid and in dilute sodium hydroxide solution. The solutions fluoresce in UV light. In the polarimeter they cause the plane of polarized light to turn to the left, thus showing optical activity .
Isomerism
The molecule is chiral; the two carbon atoms of the side chain with the HO residues are centers of chirality. In this case four stereoisomers are possible: RR , SS , RS , SR according to the Cahn-Ingold-Prelog convention . These can also be defined as two pairs of diastereomers that have the erythro or threo configuration, in analogy to the carbohydrates erythrose and threose . Naturally occurring biopterin ( L - erythro- biopterin) has the (1 R , 2 S ) configuration. Further isomers are:
- (1 S , 2 S ) - or L - threo -biopterin or orinapterin
- (1 R , 2 R ) - or D - threo -biopterin or dictyopterin
- (1 S , 2 R ) - or D - erythro -biopterin
Syntheses
The small amount of biopterin of natural origin did not allow the discoverers at that time to determine the configuration of the isolated compound. For clarification, therefore, biopterin had to be synthesized from a component in the chiral pool , the configuration of which was determined. This was found in the class of carbohydrates (monosaccharides). Thus, Patterson et al. the pterin from L - rhamnose or the chemically modified L - arabinose derivative 5-deoxy- L -arabinofuranoside and 2,4,5-triamino-3,4-dihydropyrimidin-4-one (often after its tautomer 2,4,5 -Triamino-4-hydroxypyrimidine or better known as 2,4,5-triaminopyrimidin-4-ol). L -arabinose has the erythro configuration at carbon atoms C-3 and C-4 , which consequently also had to be present in the derivative and finally in the side chain of biopterin (C-1 ', C-2'). The configuration is 1′S, 2′R ( erythro ).

reaction of 2,4,5-triamino-3,4-dihydropyrimidin-4-one with 5-deoxy- L -arabinofuranoside. The first step in the reaction is undoubtedly the formation of an N - glycoside and azomethine (Schiff's base, imine ). Since these can be formed both with the amino groups at C-4 and at C-5, positional isomers are formed; only one is shown in the picture. The next steps that involve dehydration are unclear; an Amadori rearrangement has been discussed.
However, since this condensation reaction was not selective and gave a poor yield, further syntheses were developed. From D - xylose and that was D threo diastereomer obtained which no growth-promoting effect on Crithidia fasciculata showed. Viscontini and co-workers optimized the synthesis from 5-deoxy- L -arabinofuranoside in several works. A new route from L - tartaric acid was later found for this key intermediate . Also D - ribose can be used as starting material. Further syntheses are cited at.
Biological importance
Biopterin - more precisely the redox pair 7,8-dihydrobiopterin / 5,6,7,8-tetrahydrobiopterin (the latter also abbreviated to BH 4 ) - plays an important role as a cofactor in the metabolism. In contrast to the pteridine derivatives folic acid and riboflavin , however, it can be synthesized by the human body itself and is therefore not essential . Only the tetrahydroform of biopterin is biologically active.
The biopteridine redox system is of particular importance in the oxidation of aromatic rings . Such oxidation takes place e.g. B. in the biosynthesis of the amino acid tyrosine from phenylalanine by the phenylalanine hydroxylase , in the synthesis of catecholamines in the step of the oxidation of tyrosine to L -DOPA by the tyrosine hydroxylase or in the serotonin biosynthesis in the step of the oxidation of the tryptophan to 5-hydroxytryptophan by the Tryptophan hydroxylase instead. A special feature of these oxidations is that they require the presence of molecular oxygen (see also the figure at the bottom).
Disturbances in the biopterin metabolism lead due to the importance for the metabolism of aromatic amino acids u. a. to so-called "atypical" phenylketonurias .
The nitric oxide synthase (NOS), which through several stages arginine to nitric oxide (NO) and citrulline oxidized and the alkylglycerol monooxygenase (AGMO), the ether lipids splits are also tetrahydrobiopterinabhängig.
The redox system dihydrobiopterin / tetrahydrobiopterin is comparatively complex - consider, for example, the redox systems of the cofactors NAD or FAD . A dedicated enzyme system ensures the regeneration of the oxidized form from the reduced form: pterin-4a-carbinolamine dehydratase ( EC 4.2.1.96 ) and dihydropteridine reductase ( EC 1.5.1.34 ). The following figure symbolizes the associated cycle:

Web links
Individual evidence
- ↑ a b c Entry on Biopterin. In: Römpp Online . Georg Thieme Verlag, accessed on December 5, 2011.
- ↑ a b Data sheet 6-Biopterin from Sigma-Aldrich , accessed on November 8, 2016 ( PDF ).
- ^ Entry on pteridines. In: Römpp Online . Georg Thieme Verlag, accessed on May 24, 2014.
- ↑ EL Patterson, HP Broquist, Alberta M. Albrecht, MH of Saltza, ELR Stokstad: A new pteridines in Urine required for the growth of the protozoan Crithidia fasciculata . In: J. Amer. Chem. Soc. , 77, 1955, pp. 3167-3168, doi: 10.1021 / ja01616a096 .
- ^ A b E. L. Patterson, MH von Saltza, ELR Stokstad: The Isolation and Characterization of a Pteridine Required for the Growth of Crithidia fasciculata . In: J. Amer. Chem. Soc. , 78, 1956, pp. 5871-5873, doi: 10.1021 / ja01603a044 .
- ↑ HS Forrest, HK Mitchell. In: J. Amer. Chem. Soc. , 77, 1955, p. 4865.
- ↑ M. Viscontini, E. Loeser, P. Karrer, E. Hadorn: Fluorescent substances from Drosophila melanogaster . In: Helv. Chim. Acta , 38, 1955, pp. 2034-2035, doi: 10.1002 / hlca.19550380744 .
- ↑ M. Viscontini, E. Loeser, P. Karrer: Fluorescent substances from Drosophila melanogaster. Isolation and properties of pteridine HB 2 . In: Helv. Chim. Acta , 41, 1958, pp. 440-446, doi: 10.1002 / hlca . 660410215 .
- ↑ A. Butenandt, H. Rembold: [The royal jelly of the honey bee. II. Isolation of 2-amino-4-hydroxy-6- (1,2-dihydroxypropyl) pteridine]. In: Hoppe-Seyler's journal for physiological chemistry. Volume 311, Numbers 1-3, 1958, pp. 79-83, ISSN 0018-4888 . PMID 13548912 , doi: 10.1515 / bchm2.1958.311.1.79 .
- ↑ External identifiers or database links for L-threo-biopterin : CAS number: 13039-82-2, PubChem : 135738580 , ChemSpider : 13628150 , Wikidata : Q27144825 .
- ↑ External identifiers or database links to D-threo-Biopterin : CAS number: 13019-52-8, PubChem : 135909519 , Wikidata : Q57742483 .
- ↑ External identifiers or database links to D-erythro-Biopterin : CAS number: 13039-62-8, PubChem : 135449517 , DrugBank : DB03886 , Wikidata : Q41793745 .
- ↑ M. Viscontini, H. Raschig. In: Helv. Chim. Acta , 41, 1958, p. 108.
- ↑ M. Viscontini, R. Provenzale. In: Helv. Chim. Acta , 52, 1969, p 1225th
- ↑ B. Schircks, JH Bieri, M. Viscontini. In: Helv. Chim. Acta , 68, 1985, p. 1639. There further syntheses of the Zurich group are given.
- ^ A b Anne-Marie Fernandez, Lucette Duhamel: Total Synthesis of L- Biopterin from L -Tartaric Acid via 5-Deoxy- L -arabinose . In: J. Org. Chem. , 61, 1996, pp. 8698-8700, doi: 10.1021 / jo961426s .
- ↑ K. Mori, H. Kikuchi. In: Liebigs Ann. Chem. , 1989, p. 1267.
- ↑ ER Werner, N. Blau, B. Thöny: Tetrahydrobiopterin: biochemistry and pathophysiology. In: The Biochemical journal. Volume 438, Number 3, September 2011, pp. 397-414, ISSN 1470-8728 . doi: 10.1042 / BJ20110293 . PMID 21867484 . (Review).
