Phenylketonuria
| Classification according to ICD-10 | |
|---|---|
| E70.0 | Classic phenylketonuria |
| E70.1 | Other hyperphenylalaninemia |
| ICD-10 online (WHO version 2019) | |
Phenylketonuria ( PKU ), syn. Følling disease , Föllingsche disease , Phenylbrenztraubensäure- oligophrenia and oligophrenia phenylpyruvica is one of the most common congenital metabolic disorders. It is an autosomal - recessive with an incidence of about 1: 8000 newborns inherited . Affected patients are unable to break down the amino acid phenylalanine , which means that it accumulates in the body and produces phenylpyruvate, phenyl acetate or phenyl lactate, which, if left untreated, leads to a severe mental development disorder with epilepsy . Certain metabolic products, the phenyl ketones , which are excreted in the urine , gave the disease its name. The disease can be detected in newborns by a simple serial examination. A low-protein diet started in good time can prevent the aforementioned symptoms and should ideally be carried out for life.
root cause
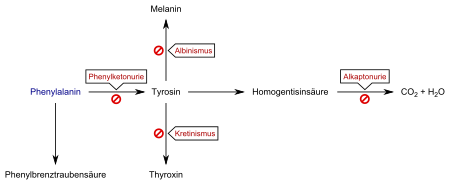
Phenylketonuria is a disorder of the amino acid metabolism. It is caused by increased concentrations of the amino acid phenylalanine, as it cannot be hydroxylated to tyrosine due to the lack of the enzyme phenylalanine hydroxylase (PAH) . Phenylalanine is an essential amino acid and must be taken in with food; A constant excess of phenylalanine leads to the severe damage described below, especially in the growing organism. It is not known why this substance has a harmful effect on brain development. It is being discussed whether the increased amount of phenylalanine will push back other amino acids in the competition for transport capacities to cross the blood-brain barrier . As a result, the body's own production of proteins ( protein biosynthesis ) in the brain is impaired. The synthesis of various messenger substances in the nervous system ( neurotransmitters ) could also be impaired. Indications for the displacement of tyrosine were found.
biochemistry
Classic phenylketonuria
In about 98% of all cases, a lack of or reduced activity of the enzyme phenylalanine hydroxylase (PAH, EC 1.14.16.1 ) leads to an accumulation of phenylalanine in the body. The alternative breakdown products phenylacetic acid (phenyl acetate), phenylpyruvic acid (phenylpyruvate, the eponymous phenylketone) and phenyllactic acid ( phenyllactate ) are increasingly excreted, which ultimately gave the disease the name phenylketonuria. Due to the lack of a metabolic pathway to tyrosine, there is a relative deficiency in this actually non-essential amino acid, which in turn must be ingested through food. Tyrosine is required for the biosynthesis of the neurotransmitter dopamine , the thyroid hormones , but also for the formation of the pigment melanin , which causes the coloring of skin and hair.
Atypical phenylketonuria
In about 2% of all cases, however, there are disorders of the metabolism of a coenzyme of phenylalanine hydroxylase, tetrahydrobiopterin (BH 4 ), and one speaks of so-called atypical phenylketonuria. Since tetrahydrobiopterin also plays a central role in the body's own production ( biosynthesis ) of the neurotransmitters serotonin and dopamine , atypical PKU usually has a more serious course. The disorders of the biopterin metabolism have different causes. On the one hand, defects in the biosynthesis of tetrahydrobiopterin are known. On the other hand, the enzyme dihydropteridine reductase (DHPR, EC 1.5.1.34 ) can have defects (dihydropteridine reductase deficiency ) . This enzyme is responsible for the reduction of dihydrobiopterin to tetrahydrobiopterin. The resulting reduced or no regeneration leads to a deficiency in the cofactor.
genetics
Classic PKU is based on a change in a gene ( point mutation ) on the long arm of chromosome 12 (12q22 to 12q24, GeneID 5053 ), which codes for phenylalanine hydroxylase. There are now known over 400 different mutations of this gene, autosomal - recessive inherited are. The extent to which this enzyme is restricted in activity depends on the type of mutation. This in turn means that the amount of protein that can be absorbed without the phenylalanine level rising above the desired range of phenylalanine tolerance varies from patient to patient.
Symptoms
Since nowadays phenylketonuria is usually found in the newborn and treated early, symptoms are rarely seen in the affected children. The abnormalities described below only occur in untreated children.
The excess of phenylalanine leads to an impairment of the brain development from the first month of life with mostly severe disturbances of the mental development, whereby the first neurological abnormalities appear in the fourth month of life. The slowing growth of the brain also leads to reduced growth of the skull, known as microcephaly . The rest of the physical development does not have to be disturbed.
The disorders described usually lead to an intellectual disability . The intelligence quotient of untreated children rarely exceeds 20. About a quarter of patients develop symptomatic epilepsy . Changes in the electrical activity of the brain can be detected in the electroencephalogram (EEG) in 75–90% of cases. Behavioral disorders with hyperactivity , aggressiveness, destructiveness, states of excitement, outbursts of anger and tendencies towards self-mutilation can also occur.
Furthermore, skin changes similar to eczema are more common than average. The lack of melanin leads to a noticeably light skin color. The children are therefore often light-blond and have blue or red eyes for the same reason. General hyperexcitability and spasticity of the muscles can occur which, in connection with damage to the brain , can lead to an atactic gait pattern . Life expectancy is apparently not restricted. Typical of the disease in food containing phenylalanine is the characteristic odor of the urine of mouse feces, which is caused in particular by phenyl acetic acid. Before the introduction of newborn screening, a diagnosis was often made based on this inherently harmless symptom.
diagnosis
The diagnosis was previously made by the symptoms mentioned and the evidence of the alternative metabolic products in the urine. Today, the search for the presence of a PKU is the prime example of a useful screening , i.e. a serial examination for the early detection of a disease. The Guthrie test used here is a simple, non-stressful, inexpensive and quick examination that leads to a clear and timely diagnosis of a serious illness with a consequent clear treatment strategy. It is crucial that all newborns are examined in the first days of life. This newborn screening has been used in Austria since 1966 and leads to 8 to 10 new diagnoses and the corresponding treatment every year. In Germany , the Guthrie test has now been replaced by tandem mass spectrometry , which enables expanded newborn screening for other disorders of the amino acid metabolism as well as various other congenital and treatable diseases.
If the result of the screening is abnormal, a column chromatographic determination of the phenylalanine concentration must be carried out as confirmation . Before starting a diet, a tetrahydrobiopterin stress test, a determination of biopterin and neopterin in the urine and a determination of the activity of the enzyme dihydropteridin reductase (DHPR) in the red blood cells ( erythrocytes ) must be carried out in order to rule out atypical PKU, as this is a different one Requires therapy.
By prenatal diagnosis can in mothers with phenylketonuria even during pregnancy by amniocentesis ( amniocentesis ) to determine whether the fetus carries the metabolic defect in itself. Since the disease is transmitted recessively , the inherently healthy father could also be the carrier of this gene mutation . In this case, if the mother were sick, the child would have a 50% risk of developing phenotypic disease. In any case, the child receives the characteristic from the mother, from the heterozygous father with a probability of 50%.
therapy
Normal mental development can be ensured if the disease is recognized and treated early on. The intake of phenylalanine through food must be strictly controlled and limited so that the phenylalanine level in the blood falls as permanently as possible to a tolerable range. In hyperphenylalaninemia, the phenylalanine levels are increased, but do not require therapy or diet. The prerequisite is regular monitoring of these values.
Classic phenylketonuria
In order to reduce the increased phenylalanine concentration as quickly as possible after the diagnosis, newborns are first given industrially manufactured, phenylalanine-free bottle feeding. If the concentration has fallen to normal values, small amounts of breast milk or commercially available bottled milk ( formula food ) are fed according to individual tolerance and the remaining amount of food is supplemented with milk that is free of phenylalanine. With the introduction of complementary foods and increasing weaning from milk formula, the diet becomes more difficult to carry out.
Since phenylalanine is a component of all dietary proteins, ultimately all protein-containing foods must be avoided. Because aspartame also contains phenylalanine, low-calorie products that have been sweetened with aspartame must always be labeled with the note “contains a source of phenylalanine”. Since even wheat and other cereal products contain a lot of vegetable protein, there are baked goods and pasta made from special low-protein flour for patients . In order to compensate for the lack of essential amino acids, the patients must also take a special amino acid mixture. A further risk of a low-phenylalanine diet is the development of a deficiency in the mineral calcium , which is important for building bones , as well as vitamin B12 and other trace elements . Apart from these dietary restrictions, life expectancy is unaffected.
The diet is best adhered to for life beyond the completion of intellectual development, as otherwise it can lead to concentration difficulties , slow reactions, changes in the EEG and swelling of the brain that can be detected by magnetic resonance imaging . Some of the patients develop spasticity of the muscles with gait disorders and tremors ( tremor ) about ten years after the end of the low-phenylalanine diet .
Enzyme therapy
In May 2018, the US Food and Drug Administration (FDA) approved pegvaliase . This is a novel, so-called enzyme therapy . Because of the risk of anaphylaxis , which most frequently occurred when the dose was up- titrated within the first year of treatment, a boxed warning is added to the label and the prescription is restricted to a limited program as part of a risk assessment and mitigation strategy (“Risk Evaluation and Mitigation Strategy ", REMS). Pegvaliase is not yet approved in Europe. In March 2018, the European approval authority EMA accepted the application for approval.
BH 4 responsiveness
About half of the European patients with classic PKU respond to a supply of tetrahydrobiopterin (sapropterin, BH 4 ), i. H. the phenylalanine level is lowered. Unlike atypical PKU, it is not a defect in the biopterin metabolism, but a property of the modified phenylalanine hydroxylase. It should be possible to tell whether someone responds to BH 4 by the type of PHA mutation.
A corresponding active ingredient called sapropterin dihydrochloride (6 R -BH 4 ), a pharmacological chaperone , has now been approved by the FDA and the EU.
Atypical phenylketonuria
Therapy for tetrahydrobiopterin deficiency depends on the cause. If the biosynthesis of BH 4 s is disturbed, the phenylalanine level can be reduced by substituting BH 4 and a low-phenylalanine diet is not necessary. However, BH 4 can not cross the blood-brain barrier sufficiently. As a result, it is again not available in sufficient quantities in the central nervous system, which hinders the biosynthesis of the neurotransmitters dopamine and serotonin . In addition to biopterin, patients are also given precursors of these neurotransmitters, L-dopa and 5-hydroxytryptophan . To adjust the individually required dose, the concentration of pterines, phenylalanine breakdown products and biogenic amines in the nerve water ( cerebrospinal fluid ) must be checked regularly . In addition, to avoid a deficiency in tetrahydrofolic acid (folinic acid), a derivative ( derivative ) of folic acid belonging to the vitamin B complex , it is also necessary to substitute this vitamin within the nervous system. Treatment with folic acid itself is more likely to worsen neurological symptoms and should therefore be avoided. In the event of a dihydropteridine reductase deficiency, it is not sufficient to take in BH 4 with the food, but a diet low in phenylalanine must also be followed.
forecast
With early treatment, the prognosis for classical phenylketonuria is extremely good. Up to the second year of life, the development of length and weight lag slightly behind compared to healthy comparison children, but later on catching up occurs and the children tend to develop overweight. The development of intelligence proceeds almost normally under early and strict diet. It largely depends on how well the treatment was followed during the first six years of life. The development of puberty and bone age do not deviate from the values of healthy children. In atypical PKU, the prospects for undisturbed development cannot be assessed quite as uniformly. On the one hand, dramatic improvements have been observed after initiation of treatment even in severely ill patients. On the other hand, symptoms of the nervous system can occur even if therapy is started early. This is more common in patients with a synthetic defect than in those with a dihydropteridine reductase deficiency .
Maternal phenylketonuria
Women with PKU and wanting to have children must follow a particularly strict diet in order not to endanger the development of the unborn child. Even a metabolically healthy child can be seriously damaged by an increased phenylalanine concentration, since the amino acid can easily migrate through the placenta . The result is an embryo-fetopathy with low birth weight, reduced head circumference (microcephalus), heart defects and other congenital malformations. In the further development, a lack of length and weight gain (failure to thrive) and a mental and motor development delay are noticeable. However, maternal phenylketonuria is not a contraindication for breastfeeding healthy children.
history
In 1934, the Norwegian doctor Ivar Asbjørn Følling was the first to detect increased excretion of phenylpyruvic acid in the urine using iron (III) chloride in mentally handicapped patients (" Følling test "). He initially called the new disease picture Imbezillitas phenylpyruvica ("Phenylpyruvic weakness"). In 1947 the actual defect in the conversion of phenylalanine to tyrosine was discovered by George A. Jervis . The next milestone in the history of PKU (also known as Fölling's disease and Oligophrenia phenylpyruvia ) was the introduction of a low-phenylalanine diet for the treatment of the disease by the German pediatrician Horst Bickel in 1953. Ten years later, the American microbiologist Robert Guthrie finally made it possible with the one he developed bacterial inhibition test the simple detection of an increased concentration of phenylalanine in the blood. The determination could be made from blood drops that were dried on filter paper, making the method also suitable for mass screening. As part of expanded newborn screening programs, the examination is now carried out in some regions with the help of tandem mass spectrometry.
literature
- Siegried A. Centerwall et al .: The discovery of phenylketonuria: the story of a young couple, two retarded children, and a scientist. In: Pediatrics. Volume 105, No. 1, Part 1, 2000, pp. 89-103. PMID 10617710 . Full text
- Friedrich K. Trefz et al., Sapropterin Study Group: Efficacy of sapropterin dihydrochloride in increasing phenylalanine tolerance in children with phenylketonuria: a phase III, randomized, double-blind, placebo-controlled study. In: The Journal of pediatrics. Volume 154, Number 5, May 2009, pp. 700-707, doi: 10.1016 / j.jpeds.2008.11.040 , PMID 19261295 .
- George A. Jervis : Studies on Phenlypyruvic Oligophrenia. The Position of the Metabolic Error. In: J. Biol. Chem. Volume 169, 1947, pp. 651-656. ( http://www.jbc.org/content/169/3/651.full.pdf PDF)
Web links
- German interest group phenylketonuria
- Forum for those affected
- Ronald D. Barley: A way out for severe metabolic disorders. Recently, phenylketonuria can no longer only be treated with a strict diet. In: Neue Zürcher Zeitung. February 16, 2011, accessed February 17, 2011 .
Individual evidence
- ↑ Screening report of the German Society for Newborn Screening ( Memento of the original from January 24, 2013 in the Internet Archive ) Info: The archive link was inserted automatically and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ a b c d e f g h i j k l A. C. Muntau et al: Phenylketonuria and hyperphenylalaninemia. In: Monthly Pediatrics. 2000, 148, pp. 179-193.
- ↑ C. Landvogt, E. Mengel u. a .: Reduced cerebral fluoro-L-dopamine uptake in adult patients suffering from phenylketonuria. In: Journal of Cerebral Blood Flow and Metabolism . Volume 28, Number 4, April 2008, pp. 824-831. doi: 10.1038 / sj.jcbfm.9600571 . PMID 17971791 .
- ↑ FDA approves a new treatment for PKU, a rare and serious genetic disease , PM FDA of May 24, 2018, accessed June 14, 2018
- ↑ BioMarin Receives Standard Approval for Palynziq ™ (pegvaliase-pqpz) Injection for Treatment of Adults with Phenylketonuria (PKU), a Rare Genetic Disease , PM BioMarin of May 24, 2018, accessed June 14, 2018
- ↑ MR Zurflüh, J. Zschocke, M. Lindner et al.: Molecular genetics of tetrahydrobiopterin-responsive phenylalanine hydroxylase deficiency . In: Hum. Mutat. tape 29 , no. 1 , January 2008, p. 167-175 , doi : 10.1002 / humu.20637 .
- ↑ L. Wang, S. Surendran, K. Michals-Matalon et al: Mutations in the regulatory domain of phenylalanine hydroxylase and response to tetrahydrobiopterin . In: Genet. Test. tape 11 , no. 2 , 2007, p. 174–178 , doi : 10.1089 / gte.2006.0520 .
- ^ Følling A. On excretion of phenylpyruvic acid in the urine as a metabolic abnormality in connection with impropriety. Hoppe-Seyler’s Z Physiol Chem 1934; 227: 169-176
- ↑ George A. Jervis: Studies on Phenylpyruvic Oligophrenia. The Position of the Metabolic Error. In: J. Biol. Chem. Volume 169, 1947, pp. 651-656. http://www.jbc.org/content/169/3/651.full.pdf
- ↑ Ludwig Weissbecker: Oligophrenia phenylpyruvica (Fölling's disease). In: Ludwig Heilmeyer (Ed.): Textbook of internal medicine. Springer-Verlag, Berlin / Göttingen / Heidelberg 1955; 2nd edition ibid. 1961, p. 1113 f.
- ^ AC Muntau, S. Beblo, B. Koletzko: Historical aspects. In: Pediatrics. 148, 2000, p. 179.