Neopterin
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
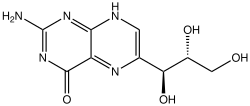
|
||||||||||||||||
| Simplified structure without stereochemistry | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Neopterin | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 9 H 11 N 5 O 4 | |||||||||||||||
| Brief description |
pale yellow crystals |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 253.21 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Neopterin is a signal messenger that is produced by special cells of the immune system ( macrophages ).
chemistry
Neopterin was first isolated from urine by Sir Frederick Gowland Hopkins in 1889 , but the structure could only be elucidated much later.
In addition to other pteridine derivatives, a new substance from the class of pteridines, for which Heinz Rembold suggested the name neopterin (Greek neo = new), was isolated from pupae of the honeybee ( Apis mellifera ) by means of chromatography, along with other pteridine derivatives . Rembold also recognized the correct structural formula and proved it through synthesis.
Neopterin contains two carbon atoms with dissimilar substituents and thus two centers of chirality . There are therefore four stereoisomers of the molecule. The D - erythro configuration was proven for the substance from the honey bee . Initially, only the D - erythro form with the absolute configuration (1 S , 2 R ) was called neopterin. Later, in the human and primate - urine in addition to the D - erythro form for that purpose enantiomeric (1 R , 2 S ) form, the ( L - erythro form) found.
Biochemical significance
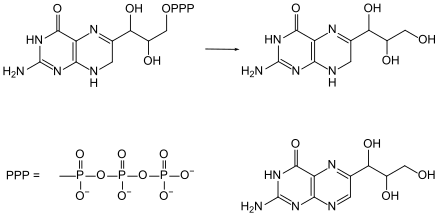
For a long time, neopterin was viewed as a precursor to the biosynthesis of biopterin . It was later discovered that the formation of neopterin is likely to be a side channel of biopterin synthesis that branches off from dihydroneopterin triphosphate . Like all other natural pteridines , neopterin is produced biosynthetically from guanosine triphosphate . With the aid of the enzyme GTP cyclohydrolase I, this is converted into dihydroneopterin triphosphate , from which the alcohol 7,8-dihydroneopterin and its dehydrogenation product , neopterin, can be formed. This arose, for example, when treating dihydroneopterin triphosphate with iodine and alkaline phosphatase .
Neopterin is an indicator of the activation of the cellular defense and an indication that a disease process is taking place in the body. Also in autoimmune diseases neopterin levels are elevated, as activated T lymphocytes , among other interferon pour -y, which activates macrophages. The pteridine compound (see pyrimidine ) can be determined in urine, blood and cerebrospinal fluid . In the normal state, the proportion in the blood serum is less than 10 nmol / l.
Neopterin is one of the best prognostic markers in patients with HIV infection, at cardiovascular risk and in various malignant tumors.
The neopterin level is determined in:
- Early detection of rejection reactions in organ transplants
- Early detection of opportunistic infections in HIV patients
- as a risk factor in coronary heart disease
- Rheumatoid arthritis
- Systemic lupus erythematosus SLE
- Immunodiagnostics in chronic fatigue syndrome (measurement of the neopterin level)
- Inflammatory stomach ( Helicobacter pylori ) and intestinal diseases ( ulcerative colitis , Crohn's disease )
- Sarcoid
- multiple sclerosis
- Lyme disease
- Hyperphenylalaninemia
- Screening of blood reserves to reduce the diagnostic window
Web links
Individual evidence
- ↑ a b c Entry on neopterin. In: Römpp Online . Georg Thieme Verlag, accessed on March 10, 2011.
- ↑ a b Data sheet D - (+) - Neopterin from Sigma-Aldrich , accessed on April 15, 2011 ( PDF ).
- ↑ Heinz Rembold, Lothar Buschmann: Investigations on the pteridines of the bee pupa (Apis Mellifica). In: Justus Liebig's Annals of Chemistry. 662, 1963, pp. 72-82 doi : 10.1002 / jlac.19636620108 .
- ↑ a b H. Rembold, L. Buschmann: Structure and synthesis of neopterin. In: Chemical Reports . 96, 1963, pp. 1406-1410 doi : 10.1002 / cber.19630960532 .
- ↑ S.-I. Takikawa, H.-C. Curtius, U. Redweik, W. Leimbacher, S. Gisla: Biosynthesis of tetrahydrobiopterin. In: European Journal of Biochemistry (1986), Vol. 161, pp. 295-302. doi : 10.1111 / j.1432-1033.1986.tb10446.x
- ↑ a b Q. Le Van, G. Katzenmeier, B. Schwarzkopf, C. Schmid, A. Bacher: Biosynthesis of biopterin studies on the mechanism of 6-pyruvoyltetrahydropteridine synthase, In: Biochemical and Biophysical Research Communications (1988), Vol. 151, pp. 512-517. ISSN 0006-291X , doi : 10.1016 / 0006-291X (88) 90623-7 .
- ↑ www.neopterin.net: Record the degree of activation of the immune system (PDF; 883 kB).