GTP cyclohydrolase I
| GTP cyclohydrolase I | ||
|---|---|---|

|
||
| Ribbon model of the GCH1 decamer according to PDB 1FB1 | ||
| Properties of human protein | ||
| Mass / length primary structure | 250 amino acids | |
| Secondary to quaternary structure | Homodecamer (2 * 5) | |
| Cofactor | zinc | |
| Isoforms | 4th | |
| Identifier | ||
| Gene names | GCH1 ; GTPCH1; GCH | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 3.5.4.16 , hydrolase | |
| Response type | Hydrolysis of two CN bonds and isomerization | |
| Substrate | GTP + H 2 O | |
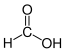
| Products | 7,8-dihydroneopterin-3'-triphosphate + formic acid | |
| Occurrence | ||
| Homology family | GTPCH1 | |
| Parent taxon | Creature | |
| Orthologue | ||
| human | House mouse | |
| Entrez | 2643 | 14528 |
| Ensemble | ENSG00000131979 | ENSMUSG00000037580 |
| UniProt | P30793 | Q05915 |
| Refseq (mRNA) | NM_000161 | NM_008102 |
| Refseq (protein) | NP_000152 | NP_032128 |
| Gene locus | Chr 14: 54.84 - 54.9 Mb | Chr 14: 47.15 - 47.19 Mb |
| PubMed search | 2643 |
14528
|
GTP cyclohydrolase I (GCH1) is the name of the enzyme that breaks down GTP into a precursor of biopterin and is thus involved in the formation of biopterin, a coenzyme . Variants of the enzyme exist in many living things, and four isoforms are known in humans, only one of which has enzymatic activity. Mutations in the GCH1 gene can cause the atypical, severe form of phenylketonuria and a form of dystonia that is treatable with levodopa .
Catalyzed equilibrium
The hydrolysis of GTP produces 7,8-dihydroneopterin-3'-triphosphate and formic acid .
GCH1 is responsible for the hydrolysis of guanosine triphosphate (GTP), from which it forms a degradation product through the catalysis of three reactions, which is converted to tetrahydrobiopterin by two other enzymes .
literature
- D. Voet, JG Voet (2004): Biochemistry (3rd ed.). John Wiley & Sons, ISBN 0-471-39223-5