Pteridines

As pteridines derivatives is called the Stammheterocyclus pteridine . This includes biologically important molecules such as folic acid and biopterin . A benzo-fused pteridine structure is found in the flavins , e.g. B. in riboflavin (vitamin B 2 ), flavin mononucleotide (FMN), flavin adenine dinucleotide (FAD).
Occurrence

The pigments on the wings of some butterflies ( pterins ) and, with a high degree of probability, dyes in the plumage of penguins (Spheniscidae, Spheniscine ) also contain the pteridine structure.
Syntheses
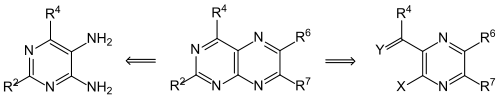
Pteridines can be prepared from pyrimidines by adding the pyrazine ring or - vice versa - from pyrazines. A retrosynthetic consideration shows that 5,6-diaminopyrimidines (see left formula; correct numbering: 4,5-diaminopyrimidines) are logical precursors. Most pteridine syntheses follow this principle, since the diaminopyrimidines are easy to prepare. In addition to the parent substance pteridine , numerous 2-, 4-, 6- and 7-substituted derivatives could be obtained. Oskar Isay was the first to synthesize 6,7-diphenylpteridine from 1,2-diphenylethanedione (benzil) and 4,5-diaminopyrimidine (in the formula for R 2 = R 4 = H, R 6 = R 7 = C 6 H 5 ). Heterocycle chemists therefore call this reaction principle the Isay reaction , but actually it is nothing more than the two-fold formation of a “Schiff base” ( azomethine ). The historically first synthesis of a pterin, leucopterin , is also based on this principle.
The second synthesis principle (right formula) was used in the synthesis of 2-amino-4-hydroxypteridine ( pterin ) (R 4 = OCH 3 , X = Cl, Y = O).
Some 4,5-diaminopyrimidines can be prepared from 4-amino-5-nitroso-pyrimidines by reduction. These can provide directly substituted pteridines by condensation with ketones in the presence of a base . This synthesis is often referred to as the Timmis reaction , after the discoverer.
Further syntheses are given for the pterins and the individual compounds.
literature
- Theophil Eicher , Siegfried Hauptmann : Chemistry of the heterocycles. Structure, reactions and syntheses. Thieme, Stuttgart 1994, ISBN 3-13-135401-1 , pp. 425-430.
- Theophil Eicher, Siegfried Hauptmann: The Chemistry of Heterocycles: Structure, Reactions, Syntheses, and Applications: Structure, Reactions, Synthesis, and Applications. 2nd Edition. Wiley-VCH Verlag, Weinheim 2003, ISBN 3-527-30720-6 , pp. 426-427.
- Wolfgang Pfleiderer : Recent Developments in Pteridine Chemistry. In: Angew. Chem. 75, pp. 993-1014 (1963). doi : 10.1002 / anie.19630752102 .
Individual evidence
- ↑ Peter Nuhn: Naturstoffchemie. S. Hirzel Wissenschaftliche Verlagsgesellschaft, Stuttgart 1990, ISBN 3-7776-0473-9 , p. 361.
- ↑ Robert Purrmann: About the wing pigments of butterflies. VII. Synthesis of Leucopterin and Nature of Guanopterin. In: Justus Liebig's Annals of Chemistry . 544 (1940), pp. 182-190, doi : 10.1002 / jlac.19405440111 .
- ^ Robert Purrmann: Constitution and synthesis of the so-called anhydroleukopterin. About the wing pigments of the butterflies XII. In: Justus Liebig's Annals of Chemistry. 548 (1941), pp. 284-292, doi : 10.1002 / jlac.19415480121 .
- ↑ Antje Findeklee: Unique yellow. Report at Spektrum.de from March 20, 2013.
- Jump up ↑ Daniel B. Thomas, Cushla M. McGoverin, Kevin J. McGraw, Helen F. James, Odile Madden: Vibrational spectroscopic analyzes of unique yellow feather pigments (spheniscins) in penguins (abstract). In: JR Soc. Interface. 6, March 20, 2013, Vol. 10 (83), doi : 10.1098 / rsif.2012.1065 .
- ^ Adrien Albert, DJ Brown, Gordon Cheeseman: Pteridine studies. Part I. Pteridines, and 2- and 4-amino- and 2- and 4-hydroxy-pteridines. In: J. Chem. Soc. 1951, pp. 474-485, doi : 10.1039 / JR9510000474 .
- ↑ O. Isay: A synthesis of purine. In: Reports of the German Chemical Society. 39 (1906), pp. 250-265, doi : 10.1002 / cber.19060390149 .
- ↑ Theophil Eicher, Siegfried Hauptmann: Chemie der Heterocyclen. Structure, reactions and syntheses. Thieme, Stuttgart 1994, ISBN 3-13-135401-1 , pp. 425-430.
- ^ GPG Dick, HCS Wood: Pteridine derivatives. Part I. A new synthesis of 2-amino-4-hydroxypteridines. In: Journal of the Chemical Society (Resumed). 1955, p. 1379, doi : 10.1039 / JR9550001379 .
- ↑ Theophil Eicher, Siegfried Hauptmann: Chemie der Heterocyclen. Structure, reactions and syntheses. Thieme, Stuttgart 1994, ISBN 3-13-135401-1 , pp. 425-430.
- ^ GM Timmis: A New Synthesis of Pteridines. In: Nature. 164 (1949), p. 139, doi : 10.1038 / 164139a0 .