Pteridine
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||
| General | ||||||||||
| Surname | Pteridine | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 6 H 4 N 4 | |||||||||
| Brief description |
yellow crystals, sublimable |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 132.12 g · mol -1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
140 ° C |
|||||||||
| boiling point |
125-130 ° C (27 k Pa ) |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Pteridine is a dinuclear, aromatic heterocycle that contains the structural elements of a pyrazine and a pyrimidine ring. The substance is not of great importance in chemistry, but the constitutional formula is the stem structure of the pterins , which includes pigments in the wings of some butterflies ( Pieridae ), etc. a. the Whites Large White ( Pieris brassicae ), rape white butterfly ( Pieris napi ) and Zitronenfalter ( Gonepteryx rhamni ). The names pterin and pteridin are derived from the Greek pteron , 'wing' , at the suggestion of Heinrich Otto Wieland . The folic acid containing the pteridine or pterin heterocycle.
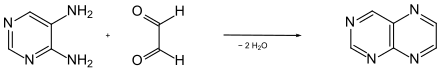
Manufacturing
A simple synthetic approach to pteridine consists in the condensation of 4,5-diaminopyrimidine with glyoxal .
literature
- Theophil Eicher, Siegfried Hauptmann: Chemistry of the heterocycles. Structure, reactions and syntheses , pp. 425-430, Thieme, Stuttgart, 1994. ISBN 3-13-135401-1 .
- Wolfgang Pfleiderer: Recent Developments in Pteridine Chemistry , Angew. Chem. 75 , 993-1014 (1963), doi : 10.1002 / anie.19630752102 .
Individual evidence
- ↑ a b c d Entry on pteridines. In: Römpp Online . Georg Thieme Verlag, accessed on May 29, 2014.
- ^ Adrien Albert, DJ Brown, Gordon Cheeseman: Pteridine studies. Part I. Pteridines, and 2- and 4-amino- and 2- and 4-hydroxy-pteridines , J. Chem. Soc. , 1951 , 474-485; doi : 10.1039 / JR9510000474 .
- ↑ RC West (Ed.) CRC Handbook of Chemistry and Physics, 59th Ed., CRC Press, Palm Beach, 1978-1979.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Robert Purrmann: About the wing pigments of butterflies. VII. Synthesis of Leucopterin and Nature of Guanopterin . Justus Liebig's Annalen der Chemie , 544 , 182-190 (1940), doi : 10.1002 / jlac . 19405440111 .
- ^ Robert Purrmann: Constitution and synthesis of the so-called anhydroleukopterin. About the wing pigments of the butterflies XII . Justus Liebigs Annalen der Chemie , 548 , 284-292 (1941); doi : 10.1002 / jlac.19415480121 .
- ↑ Clemens Schöpf, Rolf Reichert: To the knowledge of the Leukopterins , Justus Liebigs Annalen der Chemie , 548 , 82–94 (1941), doi : 10.1002 / jlac.19415480108 .
- ^ Adrien Albert, DJ Brown, Gordon Cheeseman: Pteridine studies. Part I. Pteridines, and 2- and 4-amino- and 2- and 4-hydroxy-pteridines , J. Chem. Soc. , 1951 , 474-485; doi : 10.1039 / JR9510000474 .