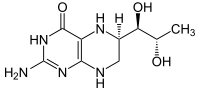
Sapropterin
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||
| General | |||||||||||||
| Non-proprietary name | Sapropterin | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 9 H 15 N 5 O 3 | ||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| Drug information | |||||||||||||
| ATC code | |||||||||||||
| properties | |||||||||||||
| Molar mass | 241.25 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
225–230 ° C (dihydrochloride) |
||||||||||||
| solubility |
good in water (25 g l −1 , dihydrochloride) |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Sapropterin is the international non- proprietary name for 5,6,7,8-tetrahydrobiopterin ( BH 4 , THB , trade name Kuvan). This reduced biopterin from the chemical group of pterins occurs naturally in higher organisms as a cofactor for the enzyme phenylalanine hydroxylase and is used as a medicinal substance, for which it is synthetically produced.
Tetrahydrobiopterin (natural substance)
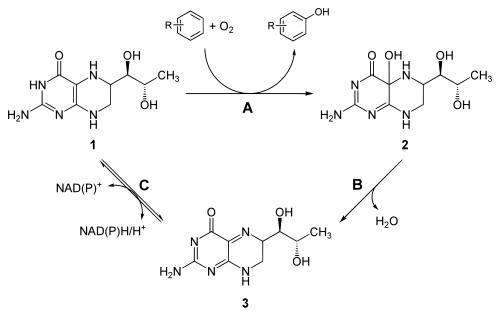
The natural tetrahydrobiopterin (BH 4 ) is probably found in every body cell in higher organisms and is an essential cofactor for a number of enzymatic processes. Tetrahydrobiopterin is produced in the cells from guanosine triphosphate in three enzymatic steps . The enzymes GTP cyclohydrolase I , 6-pyruvoyl tetrahydropterin synthase and sepiapterin reductase are used.
It acts as a cofactor in the conversion of phenylalanine to tyrosine , catalyzed by phenylalanine hydroxylase , in tyrosine hydroxylase in the conversion of tyrosine into levodopa , in tryptophan hydroxylase in tryptophan in 5-hydroxytryptophan and in NO synthase in the synthesis of nitric oxide.
Sapropterin (medicinal substance)
indication
Sapropterin is approved for the treatment of hyperphenylalaninemia (HPA) in adults and children four years and older with phenylketonuria (PKU) who are responsive to such treatment. It is also approved for the treatment of HPA in adults and children with tetrahydrobiopterin deficiency who respond to such treatment. About 30 to 50 percent of patients with PKU respond to sapropterin.
Mechanism of action
Sapropterin reduces blood phenylalanine levels in PKU patients who are responding to therapy. The mechanism underlying this effect is not yet fully understood. Saproterin acts as a pharmacological chaperone . Obviously, the accumulation and stabilization of the mutated phenylalanine hydroxylase plays an important role in those affected. This means that more phenylalanine can be broken down.
Pharmacological data
After oral ingestion of sapropterin, the maximum plasma concentration is reached after about 3 to 4 hours. In healthy adults, the plasma half-life is approximately four hours. In patients suffering from PKU and responding to sapropterin, it is 6.7 hours.
Side effects
The most common side effects are headache and rhinorrhea (nasal discharge) (10%), as well as cough, nausea, pharyngolaryngeal pain, nasal congestion, diarrhea, vomiting and abdominal pain (1 to 10%).
Pharmaceutical and chemical information
Sapropterin is in the form of its di- hydrochloride salt used for the production of drugs and orally ingested.
Sapropterin is the only drug that is available as an approved finished drug for the treatment of phenylketonuria. In the USA it was approved on December 13, 2007. On December 2, 2008, the European Commission approved the product . It has been available on the German market since April 2009.
brand names
Sapropterin is sold in Europe by Merck Serono under the brand name Kuvan ® ; in the USA by BioMarin Pharmaceutical and in Japan by Asubio Pharmaceuticals - each under the same brand name. It was previously branded as Phenoptin.
literature
- EMEA: Summary of Product Characteristics. (PDF; 79 kB)
- N. Blau et al .: Optimizing the use of sapropterin (BH (4)) in the management of phenylketonuria. In: Mol Genet Metab 96, 2009, pp. 158-163. PMID 19208488 .
Web links
- Kuvan RxList.com (English)
- Public Assessment Report (EPAR) of the European Medicines Agency (EMA) on: Sapropterin
Individual evidence
- ↑ a b Datasheet Tetrahydrobiopterin, Dihydrochloride (PDF) from Calbiochem, accessed on December 9, 2015.
- ↑ a b Data sheet (6R) -5,6,7,8-Tetrahydrobiopterin dihydrochloride from Sigma-Aldrich , accessed on April 22, 2011 ( PDF ).
- ↑ B. Thöny et al. (2000): Tetrahydrobiopterin biosynthesis, regeneration and functions. In: Biochem J . 347, pp. 1-16, PMID 10727395 .
- ↑ a b Merck Serono receives approval recommendation for Kuvan (R) in Europe. ( Memento from June 18, 2009 in the Internet Archive ) Merck Serono press release, September 26, 2008.
- ↑ B. Wick-Urban: Eating normally despite ketonuria. In: Pharmazeutische Zeitung Online 15, 2007.
- ↑ SW Gersting, M. Staudigl u. a .: Activation of phenylalanine hydroxylase induces positive cooperativity toward the natural cofactor. In: Journal of Biological Chemistry . Volume 285, Number 40, October 2010, pp. 30686-30697, doi : 10.1074 / jbc.M110.124016 . PMID 20667834 . PMC 2945563 (free full text).
- ↑ K. Michals-Matalon: Sapropterin dihydrochloride, 6-RL-erythro-5,6,7,8-tetrahydrobiopterin, in the treatment of phenylketonuria. In: Expert Opin Investig Drugs 17, 2008, pp. 245-251, PMID 18230057 .
- ^ A b M. Sanford and GM Keating: Sapropterin: a review of its use in the treatment of primary hyperphenylalaninaemia. In: Drugs 69, 2009, pp. 461-476, PMID 19323589 .
- ↑ Sapropterin: Approved by the FDA. dated January 10, 2008, accessed July 4, 2009.
- ↑ Sapropterin. Accessed online on July 4, 2009 in: Pharmazeutische Zeitung .