Phenylalanine hydroxylase
| Phenylalanine hydroxylase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 452 amino acids | |
| Secondary to quaternary structure | Homodimer | |
| Cofactor | Fe 2+ , tetrahydrobiopterin | |
| Identifier | ||
| Gene name | PAH | |
| External IDs | ||
| Enzyme classification | ||
| EC, category | 1.14.16.1 , monooxygenase | |
| Substrate | L-phenylalanine + tetrahydrobiopterine + O 2 | |
| Products | L-tyrosine + 4a-hydroxytetrahydrobiopterin | |
| Occurrence | ||
| Homology family | Phenylalanine hydroxylase | |
| Parent taxon | Creature | |
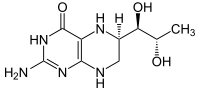
The phenylalanine hydroxylase (PAH) is that enzyme , which in all living things the conversion of L- phenylalanine to tyrosine catalyzed . It is therefore indispensable for all eukaryotes for whom high concentrations of phenylalanine in the organism are harmful. PAH contains iron , although the iron is not bound to heme , and binds tetrahydrobiopterin as a cofactor . One oxygen atom from an O2 molecule is bound for the OH group in tyrosine and the other in tetrahydrobiopterine ( see mixed-functional monooxygenases ).
In humans, it occurs mainly in the cell fluid (the cytosol ) of liver cells . Mutations in the PAH - gene can be reduced or lacking activity of the enzyme and according to hyperphenylalaninaemia for up phenylketonuria lead.
Catalyzed reaction equilibrium
Phenylalanine is oxidized to tyrosine and vice versa, tyrosine is reduced to phenylalanine.
Individual evidence
Web links
- Jennifer McDowall / Interpro: Protein Of The Month: Phenylalanine hydroxylase. (engl.)