Serotonin
| Structural formula | ||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
||||||||||||||||||||||
| General | ||||||||||||||||||||||
| Surname | Serotonin | |||||||||||||||||||||
| other names |
|
|||||||||||||||||||||
| Molecular formula |
|
|||||||||||||||||||||
| Brief description |
hygroscopic, light-sensitive crystals (serotonin hydrochloride) |
|||||||||||||||||||||
| External identifiers / databases | ||||||||||||||||||||||
|
||||||||||||||||||||||
| properties | ||||||||||||||||||||||
| Molar mass | 176.22 g mol −1 (serotonin) | |||||||||||||||||||||
| Physical state |
firmly |
|||||||||||||||||||||
| Melting point |
167–168 ° C (serotonin hydrochloride) |
|||||||||||||||||||||
| pK s value |
10.4 |
|||||||||||||||||||||
| solubility |
soluble in water (20 g l −1 at 27 ° C) and ethanol (3 g l −1 ) (serotonin hydrochloride) |
|||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||
|
||||||||||||||||||||||
| Toxicological data | ||||||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||||||||
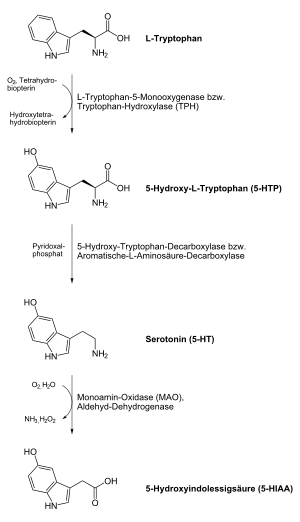
Serotonin , also 5-hydroxytryptamine ( 5-HT ) or enteramine , is a tissue hormone and neurotransmitter . It occurs in the central nervous system , the intestinal nervous system , the cardiovascular system and in the blood . The name of this biogenic amine is derived from its effect on blood pressure : Serotonin is a component of the serum that regulates the tone (tension) of the blood vessels and contributes to blood clotting . It also affects gastrointestinal activity and the transmission of signals in the central nervous system.
history

The presence of a substance in the blood that contracts blood vessels was assumed by Carl Ludwig as early as the middle of the 19th century . In the 1930s, Vittorio Erspamer isolated a substance from the mucous membrane of the gastrointestinal tract that causes the smooth muscles to contract. He called him "Enteramin". In 1948 Maurice Rapport , Arda Green and Irvine Page isolated a blood vessel-contracting substance and named it "serotonin". The structure of this substance, proposed by Maurice Rapport, was confirmed by chemical synthesis in 1951 . Shortly afterwards, Vittorio Erspamer was able to show that the enteramin he found and Rapports serotonin are identical. In 1953 Irvine Page and Betty Twarog made another important discovery with the detection of serotonin in the brain.
After the discovery of serotonin, the receptors responsible for its effects were intensively investigated. John Gaddum introduced a first distinction between "D" and "M" receptors. However, it was only with the establishment of molecular biological methods in the 1990s that it became apparent that at least 14 different serotonin receptors exist in humans , which are responsible for the diverse effects of serotonin.
Occurrence

Serotonin is widespread in nature. Even single-celled organisms such as amoebas can produce serotonin. Plants and higher mushrooms are also considered producers . In the stinging hair of the nettle , serotonin is partly responsible for its well-known effect. The plant foods richest in serotonin include walnuts , which can contain over 300 µg / g serotonin. But plantains , pineapples , bananas , kiwis , plums , tomatoes , cocoa and derived products, such as chocolate , containing more than 1 ug / g serotonin. The unusually high serotonin content, especially in walnuts, is attempted to be explained by a specific breakdown mechanism of the ammonia that is formed. Serotonin derivatives are occasionally found as a minor ingredient in psychoactive herbal drugs, such as. B. DMT , 5-MeO-DMT and Bufotenin in Yopo , a psychoactive drug from the plant Anadenanthera peregrina . For other derivatives of serotonin, such as feruloylserotonin and 4-cumaroylserotonin , a function as phytoalexin to protect against microorganisms is assumed.
In the animal kingdom, serotonin can be found in almost all species . It is one of the phylogenetically oldest neurotransmitters and, like its receptors, is already found in the nervous system as simple representatives as the nematode C. elegans . It is believed that the serotonin system, consisting of serotonin and its receptors, originated in the Precambrian over 700 million years ago.
| Body fluid | Concentration (nmol / l) |
|---|---|
| Cerebrospinal fluid | <4 |
| Platelet poor plasma | 4-15 |
| Platelet rich plasma | 1000-2500 |
| Blood serum | 300-900 |
| Whole blood | 1200 |
| urine | 900-1300 |
In the human organism, the greatest amount of serotonin occurs in the gastrointestinal tract . This is where about 95% of the body's total amount of serotonin, estimated at 10 mg, is stored. About 90% of the serotonin in the gastrointestinal tract is stored in the enterochromaffin cells , the remaining 10% can be found in the nerve cells (neurons) of the intestinal nervous system . The serotonin in the blood is distributed almost exclusively to the thrombocytes (blood platelets). This serotonin is produced by the enterochromaffin cells of the gastrointestinal tract and taken up by the platelets after it has been released into the blood. Also, basophils and mast cells can, at least in rodents , store and release serotonin. In the central nervous system, serotonin is particularly found in the neurons of the raphe nuclei . A pathologically increased production, storage and release of serotonin can often be observed in neuroendocrine tumors of the gastrointestinal tract, the carcinoids , and is responsible for their characteristic accompanying symptoms.
chemistry
Chemical properties
Like tryptamine , serotonin belongs to the group of indolamines or tryptamines . Serotonin is a weak base because of its primary aliphatic amino group . Its acidity constant pK S of 10.4 is comparable to that of the tryptamine. Therefore, under physiological conditions, serotonin is predominantly protonated . In its protonated form, serotonin is capable of fluorescence . This property is also used to prove it. The absorption maximum is 309 nm and the emission maximum is 336 nm.
synthesis
Numerous routes have been described for the synthetic production of serotonin. The basic strategies of serotonin synthesis include, in particular, the addition of the β- ethylamine component to the indole body , the hydroxylation of tryptamine and the synthesis of an indole skeleton from various possible precursor systems.
Synthesis routes based on the introduction of the β- ethylamine component start with 5-hydroxyindole , the hydroxyl group of which is either preferably protected with a benzyl group or less often with a methyl group . The former is split hydrogenolytically , the latter preferably with boron tribromide . The first laboratory synthesis, carried out by Hamlin and Fischer in 1951, uses the Mannich reaction to produce the corresponding gramin in a first synthesis step . This is then converted to chain extension with cyanide in aqueous alcohol and subsequent hydrolysis to 5-benzyloxyindole-3-acetamide. After ether cleavage and subsequent reduction of the amide , the serotonin can be isolated.
According to a publication by Merrill E. Speeter and co-workers almost at the same time , 3- (5-benzyloxy) indolyl magnesium iodide is first produced as a Grignard reagent and then reacted with chloroacetonitrile to form the corresponding 3-acetonitrile indole. The nitrile is reduced and the ether is cleaved. A variant with improved yields, which is related to the above method, describes the production of radioactively labeled [ 11 C] serotonin for positron emission tomography examinations . In this process, 5-methoxygramine is converted to the quaternary ammonium compound on N 2 and substituted with [ 11 C] hydrogen cyanide to give 5-methoxy-3-acetonitrile indole nucleophilically . This is followed by an ether cleavage and finally a reduction. One method, which is limited to two reaction steps, consists in the addition of nitroethylene to 5-benzyloxyindole with subsequent hydrogenation. The nitrovinylindole, which occurs as an intermediate, is alternatively accessible via an aldol condensation of the indole-3-carbaldehyde with nitromethane . There is also the tried and tested route via Friedel-Crafts acylation with oxalyl chloride . The 5-benzyloxy-3-indole-glyoxylyl chloride obtained in this way combines with dibenzylamine to form the amide, which is reduced and debenzylated.
Another synthesis strategy is based on the hydroxylation of tryptamine to serotonin. After the amine function has been protected to form N- methoxycarbonyltryptamine, this is reduced to the corresponding indoline with triethylsilane . Oxidation of the same by means of hydrogen peroxide in the presence of sodium tungstate dihydrate forms the intermediate product 1-hydroxy- N- methoxycarbonyltryptamine. The regioselective nucleophilic hydroxylation in the 5-position succeeds with 85% formic acid . Final hydrolysis steps such as alkaline deprotection provide the serotonin.
Another manufacturing process is based on an indole synthesis as the final step. 2,5-Dimethoxybenzaldehyde is subjected to the Knoevenagel reaction with cyanoacetate and hydrocyanic acid is added to the Michael acceptor . The succinonitrile compound is converted by hydrogenation and ether cleavage to 1,4-diamino-2- (2,5-dihydroxyphenyl) butane, which is then oxidatively converted into the end product with ferricyanide . This final step is likely to take place via the quinone , which condenses intramolecularly to the imine and aromatizes to the indole.
Apart from the mentioned paths, the structure of a tryptolin -1-one has been described, the ring opening of which produces serotonin. There is also the structure of an initially aliphatic bicyclic compound, which is later aromatized to indole.
Biochemistry and pharmacokinetics
admission
After oral administration, about 75% of serotonin is absorbed into the bloodstream and later excreted in the urine after metabolism . Similar values are found for the absorption of serotonin from foods such as bananas.
biosynthesis
In humans and animals, serotonin is built up from the amino acid L- tryptophan in a two-step reaction with the participation of the enzymes tryptophan hydroxylase and aromatic- L- amino acid decarboxylase . In the first step, the non-proteinogenic amino acid 5-hydroxytryptophan (5-HTP) is produced as an intermediate . The second step is decarboxylation to the end product serotonin. The main production sites are the enterochromaffin cells of the intestinal mucosa . From here, the serotonin produced is transported via the blood platelets. However , it is not possible to cross the blood-brain barrier , so that serotonin must also be produced in the central nervous system . The importance of neuronal serotonin production is shown by the presence of a second paralogous tryptophan hydroxylase in the brain, which arose from the first isoform through gene duplication even before the development of vertebrates .
The biosynthesis of serotonin in plants differs in the order of its steps from that in animals. With the participation of tryptophan decarboxylase, L- tryptophan is decarboxylated to the intermediate product tryptamine in the first step . In the second step, hydroxylation takes place with the help of tryptamine-5-hydroxylase to the end product serotonin.
Dismantling
After its release into the synaptic gap , the serotonin of the nerve cells is actively reabsorbed and reused with the help of the serotonin transporter (SERT), a transport protein. The serotonin produced by the enterochromaffin cells is bound quickly after its release via the same transport protein in the epithelial cells of the intestinal mucosa and in the platelets . The breakdown of serotonin takes place primarily via the enzyme monoamine oxidase (MAO) of type A and to a significantly lesser extent via MAO type B. The product 5-hydroxy-indolyl-acetaldehyde is further transformed by the aldehyde dehydrogenase into 5-hydroxyindolyl acetic acid (5- HIES) dismantled. 5-Hydroxyindolylacetic acid, which can be detected in the urine, is the main excretory product of serotonin.
Another metabolic pathway leads from the serotonin to the pineal - hormone melatonin , wherein after an acetylation of the amino group of serotonin with the involvement of serotonin N-acetyltransferase and acetyl coenzyme A , and subsequent methylation with the help of acetylserotonin-O-methyltransferase , and S- Adenosylmethionine melatonin is formed. This metabolic pathway is decisively regulated by controlling the enzyme activity of the acetyltransferase through daylight.
Functions

In the human organism, serotonin has a variety of effects, in particular on the cardiovascular system, the gastrointestinal tract and the nervous system. At the molecular level, the functions of serotonin are mediated by at least 14 different serotonin receptors (5-HT receptors), which are grouped into 7 families: 5-HT 1 to 5-HT 7 . The 5-HT 3 receptors consist of ion channels , all other known 5-HT receptors are G-protein-coupled receptors . Thanks to this multitude of serotonin receptors, which are also distributed depending on tissue, cell type and condition, the organism is able to react to different serotonin concentrations and to start various signal transduction pathways . These are the main cause of the often contradicting functions of serotonin in the organism. In addition, serotonin is able to control signal transduction processes intracellularly via a modification of proteins called serotonylation .
Cardiovascular system
The effects of serotonin on the heart and circulatory system are complex and include both the contraction ( contraction ) and relaxation ( relaxation ) of the smooth muscle of blood vessels. While in the human lungs and kidneys the blood vessel constricting effect is in the foreground, in the skeletal muscles the blood vessel expanding effect dominates. An injection of serotonin in the bloodstream causes a tri-phasic change in blood pressure. After an initial drop in blood pressure, blood pressure rises after a few seconds and ultimately ends in long-lasting hypotension .
The receptors 5-HT 1B , 5-HT 2A , 5-HT 2B and 5-HT 7 are mainly responsible for these effects . Depending on the blood vessel, they lead to a direct contraction (via 5-HT 1B , 5-HT 2A and 5-HT 2B ) or relaxation of the blood vessels (via 5-HT 7 ). Alternatively, blood vessels can be relaxed indirectly via the involvement of the endothelium (via 5-HT 1B and 5-HT 2B ) and a release of messenger substances that expand blood vessels, such as nitric oxide (NO).
In addition to these direct effects on the blood vessels, serotonin can control blood pressure and blood vessel tone in a complex manner via the central nervous system.
Blood clotting
Serotonin has both direct and indirect effects on blood clotting . The thrombocytes , also called blood platelets, whose aggregation is important for blood clotting, not only serve to store and release serotonin, but also carry serotonin receptors of the 5-HT 2A type themselves . Thanks to this, serotonin leads to an emptying of the granules of the platelets and strengthens the platelet aggregation caused by other messenger substances such as adenosine diphosphate or thrombin , thus promoting blood clotting. In smaller blood vessels, it also contributes to wound healing through vasoconstriction and thus through reduced blood flow .
Gastrointestinal system
Serotonin has various motor and sensory functions in the gastrointestinal tract . On the one hand, the digestive system reacts to serotonin, which is released from the enterochromaffin cells, on the other hand, serotonin acts as a neurotransmitter in the intestinal nervous system. The serotonin receptors 5-HT 3 and 5-HT 4 are particularly involved in mediating the effect of serotonin in the gastrointestinal tract .
Serotonin, which is released into the lamina propria of the intestinal mucosa, activates various serotonin receptors to activate primary neurons of the intestinal nervous system. These neurons transmit their stimuli on the one hand via exciting motor neurons with the neurotransmitter acetylcholine and on the other hand via inhibitory motor neurons with the transmitter nitric oxide . Under the interaction of both motor neurons, serotonin leads to a coordinated, downward wave of contraction and relaxation of the intestinal muscles, which is also known as peristalsis .
In its sensory function, released serotonin also leads to nausea and vomiting in the form of a reflex after a signal transmission involving the vagus nerve . With the involvement of the spinal nerves , complaints and pain are transmitted from the gastrointestinal tract to the brain.
Enterochromaffin cells are of the greatest importance as the source of serotonin in the gastrointestinal tract. They respond to increased intestinal pressure by releasing serotonin, which stimulates peristalsis. Also flavorings can activation of serotonin release from the enterochromaffin cells and thus to stimulate the movement of the bowel, intestinal motility , lead. A side effect of numerous cytostatic agents used in cancer chemotherapy , the so-called cytostatic vomiting, can be explained by a massive release of serotonin from the enterochromaffin cells.
eye
In the human eye, serotonin regulates intraocular pressure by activating various serotonin receptors via nerves which, among other things, use serotonin as a neurotransmitter. Possible mechanisms are a control of the aqueous humor production in the eye and an opening or closing of the pupil constrictor muscle Musculus sphincter pupillae . A side effect of so-called selective serotonin reuptake inhibitors (SSRI), the increased occurrence of glaucoma , can also be explained by an effect of serotonin on intraocular pressure.
Central nervous system
Serotonin, which is located in the central nervous system in the cell bodies, the somata serotoninergic nerve pathways in the raphe nuclei , whose axons radiate into all parts of the brain, directly or indirectly influences almost all brain functions. The most important functions of serotonin in the brain, which can not cross the blood-brain barrier and must therefore be formed on site, include the control or influencing of perception, sleep, temperature regulation, sensor technology, pain perception and pain processing, des Appetite, sexual behavior and hormone secretion. Serotonin functions on the one hand as a neurotransmitter in the synaptic gap , on the other hand it is diffusely released via free nerve endings and acts as a neuromodulator.
Mood
One of the most well-known effects of serotonin on the central nervous system is its effects on mood. It gives us a feeling of serenity, inner peace and contentment. It suppresses a whole range of different emotional states, in particular feelings of fear, aggressiveness, grief and feeling of hunger. From a neurochemical point of view, depressive moods can often be traced back to a lack of serotonin or its precursor, the amino acid tryptophan . An overdose , e.g. B. Serotonin reuptake inhibitors or incorrect combination of several drugs can lead to severe physical complaints, which can also be fatal, and hallucinations (so-called serotonin syndrome ).
By stimulating certain regions of the cerebral cortex , which are responsible for emotional regulation, serotonin essentially leads to an inhibition of impulsivity and aggressive behavior . In particular, serotonin receptors of the 5-HT 1A and 5-HT 1B types have an inhibitory effect. Conversely, low serotonin levels lead to increased impulsiveness and aggressiveness. The activation of 5-HT 2A receptors is responsible for the euphoric and hallucinogenic effects of serotonin agonists such as LSD .
In addition to dopamine , oxytocin and endorphins , serotonin is popularly referred to as the "happiness hormone" due to its effects on mood. Consumption of foods rich in tryptophan, such as chocolate or bananas , on the one hand leads to a mood-enhancing effect because of the tryptophan they contain, since - in contrast to serotonin - it can cross the blood-brain barrier . In addition, the ingested carbohydrates also cause an increased production and release of neurotransmitters (including serotonin) in the brain, which lead to this effect.
Sleep-wake rhythm
A possible role of serotonin in the regulation of the sleep-wake rhythm has been known since the 1950s. For a long time, the findings on the modulation of the sleep-wake rhythm by serotonin were partly contradicting. As far as we know today, serotonin essentially promotes the waking state. The serotonin-containing neurons of the raphe nuclei, which are connected to the nucleus suprachiasmaticus (SCN) of the hypothalamus , the seat of the main clock of mammals, are active during the waking state. In contrast, they reduce their activity in deep sleep , and practically cease to do so during REM sleep . At the same time, the suprachiasmatic nucleus controls, among other things, the production and release of the hormone melatonin from the pineal gland, which is involved in the timing of sleep .
appetite
Serotonin is a neurotransmitter, the release of which in the brain is indirectly related to food. One factor is the concentration of free tryptophan in the blood plasma. Carbohydrate and protein-rich food leads to an increase in tryptophan absorption in the brain via the release of insulin , which is associated with increased serotonin synthesis.
Serotonin is particularly associated with an appetite suppressant effect. Overweight people have decreased levels of tryptophan in the blood plasma and levels of serotonin in the brain. Medicinal substances that - like the selective serotonin reuptake inhibitors - increase the serotonin concentration in the brain lead to loss of appetite as a side effect. A selective activation of serotonin receptors of the subtype 5-HT 1A , which primarily control the release of serotonin as autoreceptors , leads to an increase in appetite by inhibiting the release of serotonin from the nerve endings. The actual appetite-reducing effect of serotonin is due in particular to the serotonin receptors 5-HT 1B or 5-HT 2C .
pain
Serotonin, which is released from injured nerve cells, for example, is a direct activator of a pain stimulus. Of greater importance is the effect of serotonin in increasing or weakening pain stimuli via descending serotoninergic neurons in the dorsal horn of the spinal cord .
Sexual behavior
Serotonin, which is released into the hypothalamus at the time of ejaculation , has primarily an inhibitory effect on sexual behavior and functions. Serotonin acts as an antagonist to dopamine . Medicinal substances which, like the selective serotonin reuptake inhibitors, increase the serotonin concentration in the brain can, in addition to a reduction in sexual desire in men, lead in particular to a restricted ability to erect or to inhibit ejaculation. In the experiment with mice, impaired serotonin production leads to bisexual sexual behavior, which can be reduced to heterosexual behavior through the use of serotonin. A study from 2015, however, speaks out against these data, since the mice with impaired serotonin production and the simultaneous presence of male and female animals in the experiment always preferred the females.
Temperature regulation
Serotonin is involved in the regulation of body temperature in the central nervous system. Depending on the brain area involved and the receptors involved, serotonin leads to an increase ( hyperthermia ) or a decrease in body temperature ( hypothermia ). The hypothermic effect of serotonin is associated in particular with the activation of serotonin receptors of the subtype 5-HT 7 .
Pathophysiology
Neuroendocrine tumors
Serotonin plays common in neuroendocrine tumors , ie with benign or malignant tumors with features of nerve cells ( neuro ne) and hormone-producing ( endocrine s) glands , a central role. The carcinoid , a collective term for neuroendocrine tumors of the gastrointestinal tract, is characterized by the overproduction of tissue hormones, especially serotonin. In contrast to most other tumors, the characteristic symptoms of the carcinoid are not due to a displacement of healthy tissue, but in particular to the effects of the increased serotonin level. Persistent diarrhea and abdominal cramps are the first signs of a carcinoid-related increase in serotonin levels in the gastrointestinal tract. In the event of increased serotonin production outside the gastrointestinal tract, for example after metastasis , systemic serotonin effects such as flush syndrome can be observed. If the serotonin level is increased over a longer period of time due to carcinoids, fibrosis , in particular of the tricuspid and pulmonary valves of the heart, occurs as a result of the hypertrophic effect of the serotonin .
Pulmonary hypertension
Disturbances of the serotonin system are considered to be a possible cause for the development of the rare pulmonary arterial hypertension . Drugs that inhibit the serotonin transporter and increase the free serotonin concentration can promote the development of pulmonary hypertension. A polymorphism of the serotonin transporter gene and a mutation of the serotonin receptor 5-HT 2B were found to be further possible causes of pulmonary hypertension.
migraine
Along with other neurotransmitters, serotonin plays a central role in the pathophysiology of migraines . Characteristic fluctuations in the serotonin level can be observed in the run-up to a migraine and during a migraine attack. A low serotonin level in the relevant brain areas is associated with the spread of so-called trigeminovascular pain stimuli as the cause of migraines.
depression
In 1969, Alec Coppen , Izyaslav Lapin and Gregory Oxenkrug simultaneously hypothesized that a lack of serotonin was the cause of depression. It is based on previous observations that the concentration of the serotonin breakdown product 5-hydroxyindolylacetic acid in the cerebral fluid is reduced in depressed patients. However, there is no clear correlation between 5-hydroxyindolylacetic acid concentration and the severity of depression, since the concentration of the metabolite is only an indirect measure of the serotonin concentration. Observations after a pharmacologically induced serotonin deficiency or the use of serotonin reuptake inhibitors support the serotonin hypothesis of depression. After the observation that in depressed patients the absorption capacity of serotonin in blood platelets and in the brain is reduced, the serotonin transporter and a genetic polymorphism of the promoter region of its gene (5-HTTLPR) was suspected as a vulnerability factor for the occurrence of depression. Since the results of scientific studies on the involvement of serotonin are partly contradicting, the serotonin hypothesis of depression is not undisputed.
pharmacology
Serotonin itself has no therapeutic application. In contrast, medicinal substances which influence the release, the effect, the reuptake and the breakdown of serotonin are used in a variety of ways for the treatment and prevention of diseases. In terms of quantity, the largest area of application of medicinal substances with an effect on the serotonin system is mental illness. Other psychotropic substances that do not have any medicinal effect also act on serotonin receptors. Some foods and dietary supplements also have effects in the brain's serotonin system.
Antidepressants
In the treatment of depression , selective serotonin reuptake inhibitors (SSRI) such as fluoxetine , fluvoxamine , paroxetine , sertraline and citalopram are very important. They are inhibitors of the serotonin transporter and lead to an increased concentration and a prolonged stay of serotonin in the synaptic gap . The effect of the older tricyclic antidepressants is based - at least in part - on an inhibition of the serotonin transporter. MAO inhibitors such as tranylcypromine and moclobemide, which are also used as antidepressants , owe their effectiveness to an inhibition of the serotonin-degrading enzyme monoamine oxidase.
Neuroleptics
The clinical properties of numerous atypical neuroleptics, which deviate from the classic neuroleptics , are explained by an additional inhibition of serotonin receptors of the subtype 5-HT 2A ( dopamine-serotonin hypothesis ) beyond the inhibition of dopamine receptors . Atypical neuroleptics such as clozapine , olanzapine and risperidone have an essentially improved effect on the negative symptoms of schizophrenia as well as a reduced frequency of extrapyramidal motor side effects and tardive dyskinesia . The ratio of the affinity of a neuroleptic for 5-HT 2A receptors to its affinity for D 2 receptors, also known as the Meltzer index , is used in this context to predict atypical neuroleptic properties.
The use of reserpine , a drug that leads to a so-called “depletion” of neurotransmitters and thus, among other things, to a reduced concentration of serotonin in the synaptic gap, is considered a milestone in the development of modern psychiatry. Today reserpine is only of historical importance.
Hypnotics (sleeping pills)
Due to the effects of serotonin on sleep, the serotonin precursors tryptophan and 5-hydroxytryptophan are used, among other things, as mild sleep aids . They are prodrugs that can cross the blood-brain barrier after being absorbed into the body and are metabolized to serotonin in the brain, among other things. Both substances fell into disrepute in the 1990s due to contamination with the neurotoxin tryptamine-4,5-dione and the occurrence of the sometimes fatal eosinophilia-myalgia syndrome as a result.
Tranquilizers
Also buspirone , a partial agonist of the 5-HT 1A receptor, is used as a psychotropic drug used to treat generalized anxiety disorder application. In its effect, buspirone differs from other anxiety relievers ( anxiolytics ), which primarily target the receptors of the neurotransmitter γ-aminobutyric acid (GABA) rather than the serotonin system .
Appetite suppressants
The appetite suppressant effect of serotonin has been exploited in various ways for appetite suppressants . Many of these drugs, such as lorcaserin and fenfluramine , show a direct stimulating effect on the 5-HT 2C receptor, which is associated with an appetite-suppressing effect. The serious side effects of the previously used appetite suppressants Aminorex and fenfluramine, which include heart valve defects and pulmonary hypertension , are also associated with an effect on the serotonin system, in particular the activation of 5-HT 2B receptors .
Another mechanism of action of some appetite suppressants such as sibutramine is the increase in the concentration of serotonin at its receptors by inhibiting the serotonin transporter.
Migraine therapeutics
In the therapy of acute migraine attacks , 5-HT 1B / 1D receptor agonists from the group of triptans such as sumatriptan are used in particular . In migraine prophylaxis, however, serotonin antagonists such as methysergide and pizotifen were used , at least until the triumphant advance of beta blockers .
Antihypertensive drugs (blood pressure lowerers)
The 5-HT 2A antagonist ketanserin and the 5-HT 1A agonist urapidil are used as antihypertensive agents for the treatment of high blood pressure. However, their antihypertensive effect is not primarily explained by an interaction with serotonin receptors, but rather by an additional interaction with adrenoceptors .
Anticoagulants
The 5-HT 2A antagonist sarpogrelate is as platelet aggregation inhibitors employed. As such, the active ingredient is used to treat strokes, heart attacks and other circulatory disorders. Its effect is attributed to a blockade of 5-HT 2A receptors on the platelet surface and, associated therewith, an inhibition of the serotonin-mediated increase in the agglomeration of the platelets.
Antiemetics
As antiemetics for treating nausea and vomiting are Setrone as ondansetron , granisetron , tropisetron , and palonosetron used. They show their greatest effectiveness in the treatment of acute vomiting after chemotherapy of tumor diseases with moderately or highly nauseating cytostatic agents such as cisplatin . The combination therapy of palonosetron with the glucocorticoid dexamethasone is also promising for delayed vomiting of cytostatic drugs. They are also used in the treatment of postoperative nausea and vomiting .
The Setrons owe their effectiveness to an inhibition of the serotonin effect on 5-HT 3 receptors, which are found in particularly high density both in the intestinal nervous system and in the vomiting center of the brain stem . The anti-nausea effect of metoclopramide is based at least in part on an antagonistic effect on these receptors. The 5-HT 3 receptor antagonist alosetron is also used in the treatment of irritable bowel syndrome .
Prokinetics
The intestinal peristalsis stimulating effect of the prokinetics is based on a serotonin-analogous stimulation of 5-HT 4 receptors . Drugs with a prokinetic effect based on activation of 5-HT 4 receptors, such as cisapride and metoclopramide, are used to treat various gastrointestinal disorders, including abdominal pain, constipation, flatulence and vomiting. The 5-HT 4 receptor agonist tegaserod was approved for the treatment of irritable bowel syndrome in the USA, but was withdrawn from the market after a short time due to safety concerns.
The therapeutic use of flavorings, which stimulate the enterochromaffin cells to release serotonin, has been postulated as an option for the development of new drugs.
Entactogens and empathogens
As entactogens ( adjective entactogenic, “touching the inside”, from Greek en , “inside”, Latin tactus “touched”) and empathogens ( empátho ; ancient Greek ἐμπάθω , “to sympathize ”; compare empathy ; genes from ancient Greek γένεσις , génesis , “ Origin ") are psychoactive substances, under the influence of which one's own emotions are perceived more intensely. The term entactogen was introduced by the American chemist David E. Nichols in the 1980s. At that time, these substances were also used in the controversial psycholytic psychotherapy , as the patient becomes more aware of his own psyche and thus access to his subconscious is easier. Both terms are used synonymously. Many entactogens, such as B. 3,4-methylenedioxyamphetamine (MDA), 3,4-methylenedioxy- N -methylamphetamine ( MDMA ), 3,4-methylenedioxy- N -methylcathinone (bk-MDMA, MDMC) act as releasers (shedding) of the endogenous monoamine Neurotransmitters serotonin and noradrenaline , which lead to an unusually high level of these messenger substances in the brain.
Psychedelics and hallucinogens
As psychedelic (composed of ancient Greek ψυχη psyche soul "and δῆλος Délos , manifest, apparently ') psychotropic substances are referred to as potent agonists of the serotonin receptors 5-HT 2A / 2C are effective. The term emerged in 1956 from a correspondence between the psychiatrist Humphry Osmond and the writer Aldous Huxley . The Duden defines psychedelic as follows: “Changing consciousness; evoking a euphoric, trance-like state of mind ”. The package insert for the psychedelic Delysid (LSD), which came on the market in 1949, indicated the possibility of using it as a psycholytic and psychotomimetic . Text excerpt indication: “(a) In analytical psychotherapy to promote mental relaxation by releasing repressed material. (b) Experimental studies of the nature of psychosis: By ingesting Delysid, the psychiatrist himself is enabled to gain insight into the world of ideas and perceptions of psychiatric patients. ”The intoxication with psychedelic states is called trip , the clinical umbrella term of psychedelics is called hallucinogens ; possible medical applications are discussed. Psychedelics include:
- hallucinogenic tryptamines : e.g. B. Psilocybin , Dimethyltryptamine (DMT), 5-MeO-DMT , AMT , 4-HO-DIPT etc.
- hallucinogenic phenylethylamines : e.g. B. Mescaline , 2C-B , 2C-I , 25I-NBOMe , DOB , DOM , TMA , Bromo-DragonFLY etc.
- hallucinogenic ergolines : e.g. B. LSD , 1P-LSD , AL-LAD , ETH-LAD , LSH , Ergin (LSA) etc.
Mushrooms containing psilocybin contain psilocybin and psilocin
Dimethyltryptamine (DMT), a naturally occurring tryptamine - alkaloid
DOB - Blotter
Individual evidence
- ↑ a b c Entry on serotonin. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ↑ a b Chattopadhyay A, Rukmini R, Mukherjee S: Photophysics of a neurotransmitter: Ionization and spectroscopic properties of serotonin . In: Biophys J . 71, 1996, pp. 1952-1960.
- ↑ a b c d e f Entry on serotonin in the ChemIDplus database of the United States National Library of Medicine (NLM) .
- ↑ a b c data sheet Serotonin hydrochloride from Sigma-Aldrich , accessed on May 8, 2017 ( PDF ).
- ↑ C. Ludwig, A. Schmidt: The behavior of the gases which flow with the blood through the irritable mammalian muscle . In: Works from the Physiological Institute in Leipzig . 1868, p. 1-61 ( online ).
- ↑ MM Rapport, AA Green, IH Page: Serum vasoconstrictor, serotonin; isolation and characterization . In: Journal of Biological Chemistry . tape 176 , no. 3 , December 1948, p. 1243-1251 , PMID 18100415 (English).
- ↑ MM Rapport: Serum vasoconstrictor (serotonin) the presence of creatinine in the complex; a proposed structure of the vasoconstrictor principle . In: Journal of Biological Chemistry . tape 180 , no. 3 , October 1949, p. 961-969 , PMID 18139191 (English).
- ↑ a b Hamlin KE, Fischer FE: The synthesis of 5-hydroxytryptamine . In: J. Am. Chem. Soc. . 73, No. 10, 1951, pp. 5007-5008. doi : 10.1021 / ja01154a551 .
- ↑ V. Erspamer, B. Asero: Identification of enteramine, the specific hormone of the enterochromaffin cell system, as 5-hydroxytryptamine . In: Nature . tape 169 , no. 4306 , May 1952, p. 800-801 , PMID 14941051 (English).
- ↑ BM Twarog, IH Page: Serotonin content of some mammalian tissues and urine and a method for its determination . In: American Journal of Physiology . tape 175 , no. 1 , October 1953, p. 157-161 , PMID 13114371 (English).
- ↑ JH Gaddum, ZP Picarelli: Two kinds of tryptamine receptor . In: British Journal of Pharmacology and Chemotherapy . tape 12 , no. 3 , September 1957, p. 323-328 , PMID 13460238 , PMC 1509685 (free full text) - (English).
- ↑ SJ Peroutka: 5-Hydroxytryptamine receptors in vertebrates and invertebrates: why are there so many? In: Neurochemistry International . tape 25 , no. 6 , December 1994, pp. 533-536 , PMID 7894329 (English).
- ^ A b J. M. Feldman, EM Lee: Serotonin content of foods: effect on urinary excretion of 5-hydroxyindoleacetic acid . In: American Journal of Clinical Nutrition . tape 42 , no. 4 , October 1985, p. 639-643 , PMID 2413754 (English).
- ↑ K. McGowan, A. Kane, N. Asarkof, J. Wicks, V. Guerina, J. Kellum, S. Baron, AR Gintzler, M. Donowitz: Entamoeba histolytica causes intestinal secretion: role of serotonin . In: Science . tape 221 , no. 4612 , August 1983, p. 762-764 , PMID 6308760 (English).
- ↑ a b K. Kang, S. Kang, K. Lee, M. Park, K. Back: Enzymatic features of serotonin biosynthetic enzymes and serotonin biosynthesis in plants . In: Plant Signaling & Behavior . tape 3 , no. 6 , June 2008, p. 389-390 , PMID 19704574 , PMC 2634310 (free full text) - (English).
- ↑ B. Muszyńska, K. Sułkowska-Ziaja, H. Ekiert: Indole compounds in fruiting bodies of some selected Macromycetes species and in their mycelia cultured in vitro . In: Pharmacy . tape 64 , no. 7 , July 2009, p. 479-480 , PMID 19694188 (English).
- ↑ T. Herraiz: Tetrahydro-beta-carbolines, potential neuroactive alkaloids, in chocolate and cocoa . In: Journal of Agricultural and Food Chemistry . tape 48 , no. 10 , October 2000, p. 4900-4904 , doi : 10.1021 / jf000508l , PMID 11052752 (English).
- ↑ P. Schröder, C. Abele, P. Gohr, U. Stuhlfauth-Roisch, W. Grosse: Latest on enzymology of serotonin biosynthesis in walnut seeds . In: Advances in Experimental Medicine and Biology . tape 467 , 1999, pp. 637-644 , PMID 10721112 (English).
- ↑ Repke, David B .; Torres Constantino M .: The chemistry of the genus Anadenanthera . In: Anadenanthera: visionary plant of ancient South America . Haworth Herbal Press, New York 2006, ISBN 0-7890-2642-2 , pp. 95-142.
- ↑ Tanaka E, Tanaka C, Mori N, Kuwahara Y, Tsuda M: Phenylpropanoid amides of serotonin accumulate in witches' broom diseased bamboo . In: Phytochemistry . 64, No. 5, November 2003, pp. 965-969. PMID 14561512 .
- ↑ DL Chase, MR Koelle: Biogenic amine neurotransmitters in C. elegans . In: WormBook . 2007, p. 1-15 , doi : 10.1895 / wormbook.1.132.1 , PMID 18050501 (English).
- ↑ Peroutka SJ, Howell TA: The molecular evolution of G protein-coupled receptors: focus on 5-hydroxytryptamine receptors . In: Neuropharmacology . 33, No. 3-4, 1994, pp. 319-324. PMID 7984268 .
- ↑ a b c d e Chauveau J, Fert V, Morel AM, Delaage MA: Rapid and specific enzyme immunoassay of serotonin . In: Clin. Chem. . 37, No. 7, July 1991, pp. 1178-1184. PMID 1855288 .
- ↑ a b Flachaire E, Beney C, Berthier A, Salandre J, Quincy C, Renaud B: Determination of reference values for serotonin concentration in platelets of healthy newborns, children, adults, and elderly subjects by HPLC with electrochemical detection . In: Clin. Chem. . 36, No. 12, December 1990, pp. 2117-2120. PMID 2253357 .
- ↑ Kim DY, Camilleri M: Serotonin: a mediator of the brain-gut connection . In: Am. J. Gastroenterol. . 95, No. 10, October 2000, pp. 2698-2709. doi : 10.1111 / j.1572-0241.2000.03177.x . PMID 11051338 .
- ↑ Benditt EP, Wong RL, Arase M, Roeper E: 5-Hydroxytryptamine in mast cells . In: Proc. Soc. Exp. Biol. Med. . 90, No. 1, October 1955, pp. 303-304. PMID 13273431 .
- ↑ Tamir H, Theoharides TC, Gershon MD, Askenase PW: Serotonin storage pools in basophil leukemia and mast cells: characterization of two types of serotonin binding protein and radioautographic analysis of the intracellular distribution of [3H] serotonin . In: J. Cell Biol. . 93, No. 3, June 1982, pp. 638-647. PMID 7118996 . PMC 2112151 (free full text).
- ↑ a b Modlin IM, Kidd M, Latich I, Zikusoka MN, Shapiro MD: Current status of gastrointestinal carcinoids . In: Gastroenterology . 128, No. 6, May 2005, pp. 1717-1751. PMID 15887161 .
- ↑ Ek A, Witkop B: The Synthesis of Labile Hydroxytryptophan Metabolites . In: J. Am. Chem. Soc. . 76, No. 22, 1954, pp. 5579-5588. doi : 10.1021 / ja01651a001 .
- ↑ Majiama R, Kotake M: Synthetic experiments in the indole group, VI .: A new synthesis of β-indolyl-alkylamines . In: Chem. Ber. . 58, 1925, pp. 2042-2046. doi : 10.1002 / cber.19250580917 .
- ↑ Speeter ME, Heinzelmann RV, Weisblatt DI: The synthesis of the blood serum vasoconstrictor principle serotonin creatinine sulfate . In: J. Am. Chem. Soc. . 73, No. 11, 1951, pp. 5514-5515. doi : 10.1021 / ja01155a580 .
- ↑ Matzke KH, Meyer GJ, Osterholz A, Coates G, Firnau G: Synthesis of 11 C-labeled 5-hydroxytryptamine for the measurement of pulmonary endothelial cell function . In: International Journal of Radiation Applications and Instrumentation. Part A. Applied Radiation and Isotopes . 42, No. 4, 1991, pp. 401-404. doi : 10.1016 / 0883-2889 (91) 90145-Q .
- ↑ Noland WE, Hovden RA: A new synthesis of serotonin . In: J. Org. Chem. . 24, No. 6, 1959, pp. 894-895. doi : 10.1021 / jo01088a632 .
- ↑ Young EHP: The synthesis of 5-hydroxytryptamine (serotonin) and related tryptamines . In: J. Chem. Soc. . 1958, pp. 3493-3496. doi : 10.1039 / JR9580003493 .
- ↑ Speeter ME, Anthony WC: THE ACTION OF OXALYL CHLORIDE ON INDOLES: A NEW APPROACH TO TRYPTAMINES . In: J. Am. Chem. Soc. . 76, No. 23, 1954, pp. 6208-6210. doi : 10.1021 / ja01652a113 .
- ↑ Somei M, Yamada F, Kurauchi T, et al. : The chemistry of indoles. CIII. Simple syntheses of serotonin, N-methylserotonin, bufotenine, 5-methoxy-N-methyltryptamine, bufobutanoic acid, N- (indol-3-yl) methyl-5-methoxy-N-methyltryptamine, and lespedamine based on 1-hydroxyindole chemistry . In: .. Chem Pharm Bull. . 49, No. 1, January 2001, pp. 87-96. PMID 11201232 .
- ↑ Harley-Mason, Jackson AH: Hydroxytryptamines. Part I. Bufotenine, 6-hydroxybufotenine, and serotonin . In: J. Chem. Soc. . 1954, pp. 1165-1171. doi : 10.1039 / JR9540001165 .
- ↑ Abramovitch RA, Shapiro D: Tryptamines, carbolines, and related compounds. Part II. A convenient synthesis of tryptamines and β-carbolines . In: J. Chem. Soc. . 1956, p. 4589. doi : 10.1039 / JR9560004589 .
- ↑ Revial G, Jabin I, Lim S, Pfau M: Aromatization of 1,6,7,7a-Tetrahydro-2H-indol-2-ones by a Novel Process. Preparation of Key-Intermediate Methyl 1-Benzyl-5-methoxy-1H-indole-3-acetate and the Syntheses of Serotonin, Melatonin, and Bufotenin . In: J. Org. Chem. . 67, No. 7, 2002, pp. 2252-2256. doi : 10.1021 / jo0110597 .
- ↑ Feldstein A, Hoagland H, Wong KK, Oktem MR, Freeman H: MAO activity in relation to depression . In: Am J Psychiatry . 120, June 1964, pp. 1192-1194. PMID 14154756 .
- ↑ Helander A, Wikström T, Löwenmo C, Jacobsson G, Beck O: Urinary excretion of 5-hydroxyindole-3-acetic acid and 5-hydroxytryptophol after oral loading with serotonin . In: Life Sci. . 50, No. 17, 1992, pp. 1207-1213. PMID 1373788 .
- ↑ Discover EggNOG 4.5.1: A database of orthologous groups and functional annotation. In: eggnog.embl.de. Retrieved September 23, 2018 .
- ↑ Klein DC: Arylalkylamine N-acetyltransferase: "the Timezyme" . In: J. Biol. Chem. . 282, No. 7, February 2007, pp. 4233-4237. doi : 10.1074 / jbc.R600036200 . PMID 17164235 .
- ↑ MJ Millan, P. Marin, J. Bockaert, CM la Cour: Signaling at G-protein-coupled serotonin receptors: recent advances and future research directions . In: Trends in Pharmacological Sciences . tape 29 , no. 9 , September 2008, p. 454-464 , doi : 10.1016 / j.tips.2008.06.007 , PMID 18676031 (English).
- ↑ Diego J. Walther, Jens-Uwe Peter, Sandra Winter, Markus Höltje, Nils Paulmann, Maik Grohmann, Jakob Vowinckel, Victor Alamo-Bethencourt, Claudia S. Wilhelm, Gudrun Ahnert-Hilger, Michael Bader: Serotonylation of small GTPases is a signal transduction pathway that triggers platelet alpha-granule release . In: Cell . tape 115 , no. 7 , December 2003, p. 851-862 , doi : 10.1016 / S0092-8674 (03) 01014-6 , PMID 14697203 (English).
- ^ PR Saxena, A. Lawang: A comparison of cardiovascular and smooth muscle effects of 5-hydroxytryptamine and 5-carboxamidotryptamine, a selective agonist of 5-HT1 receptors . In: Archives Internationales de Pharmacodynamie et de Therapy . tape 277 , no. 2 , October 1985, p. 235-252 , PMID 2933009 (English).
- ↑ C. Ullmer, K. Schmuck, HO Kalkman, H. Lübbert: Expression of serotonin receptor mRNAs in blood vessels . In: FEBS Letters . tape 370 , no. 3 , August 1995, p. 215-221 , doi : 10.1016 / 0014-5793 (95) 00828-W , PMID 7656980 (English).
- ↑ SW Watts, ML Cohen: Vascular 5-HT receptors: pharmacology and pathophysiology of 5-HT 1B , 5-HT 1D , 5-HT 1F , 5-HT 2B and 5-HT 7 receptors . In: Neurotransmissions . tape 15 , 1999, p. 3-15 (English).
- ^ AG Ramage, CM Villalón: 5-hydroxytryptamine and cardiovascular regulation . In: Trends in Pharmacological Sciences . tape 29 , no. 9 , September 2008, p. 472-481 , PMID 19086344 (English).
- Jump up ↑ Josef T. Prchal, Marshall A. Lichtman, William A. Williams, Ernest Beutler, Kenneth Kaushansky , Thomas J. Kipps, Uri Seligsohn: Williams hematology . McGraw-Hill, New York 2006, ISBN 0-07-143591-3 (English).
- ↑ Gershon MD: Review article: serotonin receptors and transporters - roles in normal and abnormal gastrointestinal motility . In: Aliment. Pharmacol. Ther. . 20 Suppl 7, November 2004, pp. 3-14. doi : 10.1111 / j.1365-2036.2004.02180.x . PMID 15521849 .
- ↑ a b Costedio MM, Hyman N, Mawe GM: serotonin and its role in colonic function and in gastrointestinal disorders . In: Dis. Colon rectum . 50, No. 3, March 2007, pp. 376-388. doi : 10.1007 / s10350-006-0763-3 . PMID 17195902 .
- ↑ Bülbring E, Crema A: The release of 5-hydroxytryptamine in relation to pressure exerted on the intestinal mucosa . In: J. Physiol. (Lond.) . 146, No. 1, April 1959, pp. 18-28. PMID 13655213 . PMC 1356887 (free full text).
- ↑ Gershon MD: Review article: roles played by 5-hydroxytryptamine in the physiology of the bowel . In: Aliment. Pharmacol. Ther. . 13 Suppl 2, May 1999, pp. 15-30. PMID 10429737 .
- ↑ a b Braun T, Voland P, Kunz L, Prinz C, Gratzl M: Enterochromaffin cells of the human gut: sensors for spices and odorants . In: Gastroenterology . 132, No. 5, May 2007, pp. 1890-1901. doi : 10.1053 / j.gastro.2007.02.036 . PMID 17484882 .
- ↑ Minami M, Endo T, Hirafuji M, et al. : Pharmacological aspects of anticancer drug-induced emesis with emphasis on serotonin release and vagal nerve activity . In: Pharmacol Ther . . 99, No. 2, August 2003, pp. 149-165. PMID 12888110 .
- ^ A b Costagliola C, Parmeggiani F, Semeraro F, Sebastiani A: Selective serotonin reuptake inhibitors: a review of its effects on intraocular pressure . In: Curr Neuropharmacol . 6, No. 4, December 2008, pp. 293-310. doi : 10.2174 / 157015908787386104 . PMID 19587851 . PMC 2701282 (free full text).
- ↑ Sharif NA: Serotonin-2 receptor agonists as novel ocular hypotensive agents and their cellular and molecular mechanisms of action . In: Curr Drug Targets . 11, No. 8, August 2010, pp. 978-993. PMID 20426763 .
- ↑ L. Descarries, MA Audet, G. Doucet, S. Garcia, S. Oleskevich, P. Séguéla, JJ Soghomonian, KC Watkins: Morphology of central serotonin neurons. Brief review of quantified aspects of their distribution and ultrastructural relationships . In: Annals of the New York Academy of Sciences . tape 600 , 1990, pp. 81-92 , PMID 2252339 (English).
- ^ RJ Nelson, BC Trainor: Neural mechanisms of aggression . In: Nature Reviews Neuroscience . tape 8 , no. 7 , July 2007, p. 536-546 , doi : 10.1038 / nrn2174 , PMID 17585306 (English, pdf ( Memento from February 11, 2014 in the Internet Archive )). Neural mechanisms of aggression ( Memento of the original from February 11, 2014 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ DE Nichols: Hallucinogens . In: Pharmacology & Therapeutics . tape 101 , no. 2 , February 2004, p. 131-181 , doi : 10.1016 / j.pharmthera.2003.11.002 , PMID 14761703 (English).
- ↑ M. Jouvet: Sleep and serotonin: an unfinished story . In: Neuropsychopharmacology . tape 21 , 2 Suppl, August 1999, pp. 24S-27S , doi : 10.1016 / S0893-133X (99) 00009-3 , PMID 10432485 (English).
- ↑ Monti JM: The role of dorsal raphe nucleus serotonergic and non-serotonergic neurons, and of their receptors, in regulating waking and rapid eye movement (REM) sleep . In: Sleep Med Rev . 14, No. 5, October 2010, pp. 319-327. doi : 10.1016 / j.smrv.2009.10.003 . PMID 20153670 .
- ↑ Monti JM, Jantos H: The roles of dopamine and serotonin, and of their receptors, in regulating sleep and waking . In: Prog Brain Res.. . 172, 2008, pp. 625-646. doi : 10.1016 / S0079-6123 (08) 00929-1 . PMID 18772053 .
- ^ Morin LP: Serotonin and the regulation of mammalian circadian rhythmicity . In: Ann. Med. . 31, No. 1, February 1999, pp. 12-33. PMID 10219711 .
- ↑ Cardinali DP et al. : Circadian control by serotonin and melatonin receptors: Clinical relevance . In: Monti JM (Ed.): Serotonin and Sleep: Molecular, Functional and Clinical Aspects . Birkhäuser Basel, 2008, ISBN 3-7643-8560-X .
- ^ RJ Wurtman, JJ Wurtman: Brain serotonin, carbohydrate-craving, obesity and depression . In: Obesity Research . 3 Suppl 4, November 1995, p. 477S-480S , PMID 8697046 (English).
- ↑ SE Ohia, CA Opere: Obesity: epidemiology, pathophysiology, and prevention . Ed .: Harry G. Preuss, Debasis Bagchi. Taylor & Francis, Washington, DC 2007, ISBN 0-8493-3802-6 , Role of neurotransmitters in obesity regulation, pp. 71-80 (English).
- ↑ EM Hull, JW Muschamp, S. Sato: Dopamine and serotonin: influences on male sexual behavior . In: Physiology & Behavior . tape 83 , no. 2 , November 2004, p. 291-307 , doi : 10.1016 / j.physbeh.2004.08.018 , PMID 15488546 (English).
- ↑ MD Waldinger, B. Olivier: Selective serotonin reuptake inhibitor-induced sexual dysfunction: clinical and research considerations . In: International Clinical Psychopharmacology . 13 Suppl 6, July 1998, p. S27-33 , PMID 9728672 (English).
- ↑ Yan Liu, Yun'ai Jiang et al. a .: Molecular regulation of sexual preference revealed by genetic studies of 5-HT in the brains of male mice. In: Nature. 472, 2011, p. 95, doi : 10.1038 / nature09822 .
- ↑ Mariana Angoa-Pérez, Nieves Herrera-Mundo u. a .: Brain Serotonin Signaling Does Not Determine Sexual Preference in Male Mice. In: PLOS ONE. 10, 2015, p. E0118603, doi : 10.1371 / journal.pone.0118603 .
- ^ RD Myers: Serotonin and thermoregulation: old and new views . In: Journal of Physiology (Paris) . tape 77 , no. 2-3 , 1981, pp. 505-513 , PMID 6793718 (English).
- ^ PB Hedlund, JG Sutcliffe: Functional, molecular and pharmacological advances in 5-HT7 receptor research . In: Trends in Pharmacological Sciences . tape 25 , no. 9 , September 2004, p. 481-486 , PMID 15559250 (English).
- ^ Druce M, Rockall A, Grossman AB: Fibrosis and carcinoid syndrome: from causation to future therapy . In: Nature Reviews Endocrinology . 5, No. 5, May 2009, pp. 276-283. doi : 10.1038 / nrendo.2009.51 . PMID 19444261 .
- ^ Maclean MR, Dempsie Y: The serotonin hypothesis of pulmonary hypertension revisited . In: ... Adv Exp Med Biol. . 661, 2010, pp. 309-322. doi : 10.1007 / 978-1-60761-500-2_20 . PMID 20204739 .
- ↑ Eddahibi S, Morrell N, d'Ortho MP, Naeije R, Adnot S: Pathobiology of pulmonary arterial hypertension . In: Eur. Respir. J. . 20, No. 6, December 2002, pp. 1559-1572. PMID 12503718 .
- ↑ Esteve JM, Launay JM, Kellermann O, Maroteaux L: Functions of serotonin in hypoxic pulmonary vascular remodeling . In: Cell Biochem. Biophys. . 47, No. 1, 2007, pp. 33-44. PMID 17406058 .
- ↑ Hamel E: Serotonin and migraine: biology and clinical implications . In: Cephalalgia . 27, No. 11, November 2007, pp. 1293-1300. doi : 10.1111 / j.1468-2982.2007.01476.x . PMID 17970989 .
- ^ Coppen AJ: Biochemical aspects of depression . In: Int Psychiatry Clin . 6, No. 2, 1969, pp. 53-81. PMID 5817856 .
- ^ Lapin IP, Oxenkrug GF: Intensification of the central serotoninergic processes as a possible determinant of the thymoleptic effect . In: Lancet . 1, No. 7586, January 1969, pp. 132-136. PMID 4178247 .
- ↑ Dencker SJ, Malm U, Roos BE, Werdinius B: Acid monoamine metabolites of cerebrospinal fluid in mental depression and mania . In: J. Neurochem. . 13, No. 12, December 1966, pp. 1545-1548. PMID 5962034 .
- ↑ Mendels J, Frazer A, Fitzgerald RG, Ramsey TA, Stokes JW: Biogenic amine metabolites in cerebrospinal fluid of depressed and manic patients . In: Science . 175, No. 28, March 1972, pp. 1380-1382. PMID 5059569 .
- ^ Mann JJ: Role of the serotonergic system in the pathogenesis of major depression and suicidal behavior . In: Neuropsychopharmacology . 21, No. 2 Suppl, August 1999, pp. 99S-105S. doi : 10.1016 / S0893-133X (99) 00040-8 . PMID 10432495 .
- ↑ Shopsin B, Friedman E, Gershon S: Parachlorophenylalanine reversal of tranylcypromine effects in depressed patients . In: Arch. Gen. Psychiatry . 33, No. 7, July 1976, pp. 811-819. PMID 133650 .
- ↑ Lacasse JR, Leo J: Serotonin and depression: a disconnect between the advertisements and the scientific literature . In: PLoS Med. . 2, No. 12, December 2005, p. E392. doi : 10.1371 / journal.pmed.0020392 . PMID 16268734 . PMC 1277931 (free full text).
- ^ HY Meltzer: Clinical studies on the mechanism of action of clozapine: the dopamine-serotonin hypothesis of schizophrenia . In: Psychopharmacology . 99 Suppl, 1989, pp. S18-27 , PMID 2682729 (English).
- ↑ Curzon G: How reserpine and chlorpromazine act: the impact of key discoveries on the history of psychopharmacology . In: Trends Pharmacol. Sci. . 11, No. 2, February 1990, pp. 61-63. PMID 2180160 .
- ↑ Turner EH, Loftis JM, Blackwell AD: Serotonin a la carte: supplementation with the serotonin precursor 5-hydroxytryptophan . In: Pharmacol. Ther. . 109, No. 3, March 2006, pp. 325-338. doi : 10.1016 / j.pharmthera.2005.06.004 . PMID 16023217 .
- ↑ Bays HE: Lorcaserin and adiposopathy: 5-HT2c agonism as a treatment for 'sick fat' and metabolic disease . In: Expert Rev Cardiovasc Ther . 7, No. 11, November 2009, pp. 1429-1445. doi : 10.1586 / erc.09.123 . PMID 19900026 .
- ↑ Curzon G, Gibson EL, Oluyomi AO: Appetite suppression by commonly used drugs depends on 5-HT receptors but not on 5-HT availability . In: Trends Pharmacol. Sci. . 18, No. 1, January 1997, pp. 21-25. PMID 9114726 .
- ^ PA van Zwieten, GJ Blauw, P. van Brummelen: Pharmacological profile of antihypertensive drugs with serotonin receptor and alpha-adrenoceptor activity . In: Drugs . 40 Suppl 4, 1990, p. 1-8; discussion 28-30 , PMID 1982649 (English).
- ↑ T. Nagatomo, M. Rashid, H. Abul Muntasir, T. Komiyama: Functions of 5-HT2A receptor and its antagonists in the cardiovascular system . In: Pharmacology & Therapeutics . tape 104 , no. 1 , October 2004, p. 59-81 , doi : 10.1016 / j.pharmthera.2004.08.005 , PMID 15500909 (English).
- ↑ Billio A, Morello E, Clarke MJ: Serotonin receptor antagonists for highly emetogenic chemotherapy in adults . In: Cochrane Database Syst Rev . No. 1, 2010, p. CD006272. doi : 10.1002 / 14651858.CD006272.pub2 . PMID 20091591 .
- ↑ Apple CC, Ill P, Piper S, et al. : Nausea and vomiting in the postoperative period. Expert and evidence-based recommendations on prophylaxis and therapy . In: Anaesthesiologist . 56, No. 11, November 2007, pp. 1170-1180. doi : 10.1007 / s00101-007-1210-0 . PMID 17726590 .
- ↑ Rex A, Bert B, Fink H: Pharmacology of the 5-HT 3 antagonists . In: Pharm Our Time . 36, No. 5, 2007, pp. 342-353. doi : 10.1002 / pauz.200700230 . PMID 17722161 .
- ↑ Mayer EA, Bradesi S: alosetron and irritable bowel syndrome . In: Expert Opin Pharmacother . 4, No. 11, November 2003, pp. 2089-2098. doi : 10.1517 / 14656566.4.11.2089 . PMID 14596662 .
- ^ DE Nichols: Differences Between the Mechanism of Action of Mdma, Mbdb, and the Classic Hallucinogens. Identification of a New Therapeutic Class: Entactogens. In: Journal of Psychoactive Drugs. Volume 18, No. 4, 1986, pp. 305-313 (English; online at scribd.com; doi : 10.1080 / 02791072.1986.10472362 ).
- ↑ Rothman, RB & Baumann, MH (2002): Therapeutic and adverse actions of serotonin transporter substrates . In: Pharm. Ther. Vol. 95, pp. 73-88, PMID 12163129 .
- ^ DE Nichols: Hallucinogens. In: Pharmacology & therapeutics. Volume 101, Number 2, February 2004, pp. 131-181, doi : 10.1016 / j.pharmthera.2003.11.002 , PMID 14761703 (review).
- ^ Thomas S. Ray, Olivier Jacques Manzoni: Psychedelics and the Human Receptorome. In: PLoS ONE. 5, 2010, p. E9019, doi : 10.1371 / journal.pone.0009019 .
- ↑ Huxley, A., Palmer, C., and Horowitz, M. (1977). Moksha: Writings on Psychedelics and the Visionary Experience (1931-1963). New York, NY: Stonehill. TO DR. HUMPHRY OSMOND [smith 744], page 107.
- ↑ Homepage: Duden - psychedelic, more rarely psychedelic - spelling, meaning, definition, origin. In: duden.de. Retrieved February 28, 2016 .
- ↑ Albert Hofmann: LSD - my problem child. The discovery of the miracle drug, Stuttgart 1993, p. 55.
- ↑ CS Grob, AL Danforth, GS Chopra, M. Hagerty, CR McKay, AL Halberstadt, GR Greer: Pilot study of psilocybin treatment for anxiety in patients with advanced-stage cancer. In: Archives of general psychiatry. Volume 68, number 1, January 2011, pp. 71-78, doi : 10.1001 / archgenpsychiatry.2010.116 , PMID 20819978 .
- ↑ KW Tupper, E. Wood, R. Yensen, MW Johnson: Psychedelic medicine: a re-emerging therapeutic paradigm. In: CMAJ: Canadian Medical Association journal = journal de l'Association medicale canadienne. Volume 187, number 14, October 2015, pp. 1054-1059, doi : 10.1503 / cmaj.141124 , PMID 26350908 , PMC 4592297 (free full text) (review)
- ↑ Therapeutic advances in psychopharmacology. Volume 4, number 4, August 2014, pp. 156-169, doi : 10.1177 / 2045125314527985 , PMID 25083275 , PMC 4104707 (free full text) (review).
literature
- Julie G. Hensler: Serotonin . In: George J. Siegel, R. Wayne Albers, Brady Scott, Donald D. Price (Eds.): Basic neurochemistry: molecular, cellular and medical aspects . Elsevier Academic, Amsterdam 2006, ISBN 0-12-088397-X , pp. 227-248.
- Elaine Sanders-Bush, Steven E. Mayer: 5-Hydroxytryptamine (serotonin): Receptor agonists and antagonists . In: Joel G. Hardman, Lee E. Limbird, Alfred Goodman Gilman (eds.): Goodman & Gilman's The pharmacological basis of therapeutics , 10th edition, McGraw-Hill, 2001, ISBN 0-07-135469-7 , pp. 269-290.
- Paul M. Vanhoutte, PR Saxena, Rodolfo Paoletti, Nicoletta Brunello: Serotonin: from cell biology to pharmacology and therapeutics . Kluwer Academic Publishers, Boston 1993, ISBN 0-7923-2518-4 .
Web links
- Birgit Hertwig: Serotonin. In: Laborjournal Edition December 12 , 2009, accessed on September 18, 2018 .
- The biochemistry of fear . In: taz - the daily newspaper of May 23, 2008. Informative article on the new study, accessed on March 25, 2012.