Ergoline
Ergolines (also ergoline derivatives ) are chemical compounds derived from ergoline . They occur naturally in the form of ergot alkaloids . These include the water-soluble ergines (derivatives of lysergic acid and its amides ), water-insoluble ergopeptides and the group of clavins . In addition, numerous, partially synthetic Ergolines are used. By hydrogenation in the 9,10-position of naturally occurring ergot alkaloids, for example dihydroergotamine and dihydroergocryptine are obtained. Other important ergoline derivatives are characterized by substituents in the positions N 1 (e.g. in methysergide ), N 6 (e.g. in cabergoline ) and C 8 (e.g. in pergolide ). An 8,9-benz fused ergoline effective as a D 1 dopamine agonist has the code CY-208,243. Bromocriptine is an ergole brominated on the indole in position C 2 . Metabolic hydroxylation is observed in the C 13 and C 14 positions .
Ergolene
The great majority of naturally occurring ergolines have an additional double bond in the 8.9- (Δ 8.9- ergolene) or in the 9,10-position (Δ 9,10- ergolene) compared to the ergoline . The Δ 8,9 -ergolenes include paspalsic acid and the clavins Agroclavin and Elymoclavin . By far the largest naturally occurring spectrum of substances is possessed by the Δ 9,10- ergolene. In addition to lysergic acid and the simple amides derived from it (e.g. ergine ), they include numerous clavins , such as lysergol , penniclavine and setoclavine , and the therapeutically relevant ergot alkaloids of the peptide type. Some important partially synthetic ergolines, such as lisuride , bromocriptine and methysergide, also have a Δ 9,10 -ergole skeleton.
Modifications in position 1
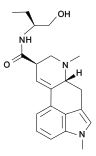 |

|
| Methysergide | Mesulengine |
 |

|
| Bromocriptine | 2-bromo-LSD |
 |

|
| Pergolide | Cabergoline |
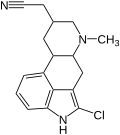 |

|
| Lergotril | Lisurid |
Chemical modifications in the N 1 position are among the most common changes made to the ergoline system. On the one hand, the indole nitrogen is easily accessible for alkylations , acylations , Mannich reactions, and Michael additions . On the other hand, animal experiments on rodent models raised the hope that N 1 -alkylation could increase the pharmacological selectivity of ergolines in favor of the serotonin receptors and thus improve the risk-benefit profile of potential therapeutics with an ergoline structure. However, the pharmacological selectivity observed later turned out to be a rodent-restricted phenomenon. Of the N 1 -modified ergolines, only methysergide in the treatment of migraines and nicergoline in the treatment of senile brain disorders achieved therapeutic importance in human medicine. Metergolin is also used in veterinary medicine. Other representatives of this group of substances include amesergide , sergolexol and mesulergin .
Modifications in position 2
With a few exceptions, all natural ergolines are unsubstituted. A striking exception is fumigaclavin C from Aspergillus fumigatus , which is formed from fumigaclavin A , which is unsubstituted in position 2, and dimethylallyl pyrophosphate .
Chemically, position 2 in the ergoline skeleton is easily accessible for electrophilic and radical substitution reactions. In addition to halogenated ergolines, 2-aminoergolines and 2-acylergolines have also been described. The brominated bromocriptine in position 2 is used in the therapy of Parkinson's disease , amenorrhea , acromegaly and to inhibit prolactin secretion . Lergotril has also been shown to be effective in clinical studies for the treatment of Parkinson's disease, but it has too unfavorable side effects for a therapeutic agent. The radiolabelable 2-bromo-LSD is an important tool in experimental pharmacology.
Modifications in item 6
All naturally occurring ergoline alkaloids are methylated at the N 6 position. With the help of Von Braun degradation using cyanogen bromide , the corresponding demethylated ergoline derivatives can be prepared. Replacing the N 6 -methyl group with longer-chain alkyl groups leads to an increase in the potency of the dopamine receptors . Propyl- and allyl-substituted ergolines in particular have proven to be particularly effective. With pergolide and cabergoline, two representatives of the N 6 -substituted ergolines were used in the therapy of Parkinson's disease .
Modifications in item 8
The substituents in position 8 of the ergoline system have a particularly broad spectrum. Since this position is prochiral in the ergoline system , a stereochemical distinction can be made between 8α- and 8β-substituted ergolines. According to the substituents in position 8, the naturally occurring ergolines, the ergot alkaloids , are divided into different groups. They include the tetracyclic representatives of the clavinal acloids, the lysergic acids and their simple amides, and the ergot alkaloids of the peptide type, including the ergopeptins .
The spectrum for substituents in position 8 is further expanded in the case of partially or fully synthetic ergolines. In the simplest case, the amide group of a natural ergoline can be exchanged. Nicergoline, sergolexole and the metergoline used in veterinary medicine have a carbamic acid ester substituent in position 8β and the anti-Parkinson drug pergolide has a methylthiomethyl group. 8β-Hydroxy- and 8β-aminoergolines have also been described. Starting from lysergol, the chain length of the substituent can be gradually lengthened with the help of potassium cyanide . Thus contributes lergotrile in position 8 is a C 2 instead of a C 1 group. The partially synthetically modified ergolines in position 8 also include the psychedelic LSD , the diethylamide of lysergic acid. In contrast to all other pharmacologically relevant ergolines, the ergolinyl urea derivatives lisuride and terguride as well as mesulergine have an 8α configuration. Although the 8β-configured ergopeptins can also be converted into the 8α-configured ergopeptinins by C 8 -pimerization, these have no therapeutic significance.
Modifications in item 9
Aspergillus species, especially Aspergillus fumigatus , are producers of clavins with a hydroxy or alkoxy substituent in position 9 of the ergoline system. Thesealkaloids, knownas fumigaclavins , have an antimicrobial effect.
Modifications in item 10
A substituent in position 10 of the ergoline backbone can be introduced by addition or substitution reactions. Under the influence of light, water is added to the double bond of Δ 9,10 -ergolen in an aqueous solution to form so-called Lumi-ergolines. In this way, 10-alkoxyergolines can also be prepared from an alcoholic solution of Δ 9,10 -ergolenes. A wider range of substituents in position 10 enables electrophilic substitution using butyl lithium. The ergolines modified in position 10 include nicergoline.
Framework modifications
Framework modifications refer both to the exchange of framework atoms for other atoms, mostly heteroatoms , and to changes in the number of members of rings A to D. Numerous substances modified in this way have been described. The name obeys the general rules of chemical nomenclature . When exchanging atoms, the exchange position is placed in front and the exchange atom is named with an affix , i.e. with aza (X → N), oxa (X → O), thia (X → S), carba (X → C) or deaza (N → C), where X stands for any skeletal atom. Example: 1-Oxa-2-azaergoline.
The naturally occurring bi- or tricyclic secoergolines are precursors of the tetracyclic ergoline alkaloids. Rings C and D are open for the clavins dimethylallyltryptophan and N -dimethylallyltryptophan. Among the C ring closed Clavinen include Chanoclavine and Paliclavin . Clavipitic acid with a tetrahydro-1 H -azepino [5,4,3- cd ] indole skeleton is formed by an alternative ring closure . Secoergolines have a comparatively low biological activity.
Synthetic modification of the ergoline skeleton showed that an intact indole partial structure is essential for the pharmacological effect. In 1980, Kornfeld and co-workers described the synthesis of indazole 2-azaergolines. They came to the conclusion that the 2-aza-clavins they examined lost the classic effects of their Indolian counterparts. Natural ergolines can be elegantly derivatized to hydroquinolino [5,6- ef ] quinazoline , benzofuro [4,3- fg ] hydroquinoline and benz isoxazolo [4,3- fg ] hydroquinoline analogues by oxidative opening of the indole ring using sodium periodate . To obtain the oxygen-containing heterocycles , the intermediate anilines are diazotized and gently hydrolyzed to phenols (cf. boiling ). Nor-6- carba- lysergic acid, in which a methylene group is located in position 6 instead of an amine nitrogen , and compounds in which the amine nitrogen of the parent compound is formally shifted to positions 7, 8 and 9 are also known in the specialist literature . An alternative biosynthetic ring closure in ring D leads to the naturally occurring 8-oxaergoline paspaclavin .
While a ring expansion in ring D is so far unknown, the narrowing of this ring, which leads to the designation D -norergoline, was realized, among other things in the form of D -nor-LSD. A formal scaffold rearrangement involving rings C and D has been described in the form of 5 (10 → 9) Abeo- ergolines. Some representatives of these compounds have been characterized as highly potent and selective 5-HT 1A receptor agonists.
Individual evidence
- ↑ Schardl CL, Panaccione DG, Tudzynski P: ergot alkaloids - biology and molecular biology . In: The Alkaloids: Chemistry and Biology . 63, 2006, pp. 45-86. doi : 10.1016 / S1099-4831 (06) 63002-2 . PMID 17133714 .
- ↑ Martin Buchta, Ladislav Cvak: Ergot alkaloids and other metabolites of the genus Claviceps . In: Vladimir Kren, Ladislav Cvak (eds.): Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . CRC Press, 2004, ISBN 0203304195 , pp. 173-201.
- ↑ a b c Petr Bulej, Ladislav Cvak: Chemical modifications of ergot alkaloids . In: Vladimir Kren, Ladislav Cvak (eds.): Ergot: The Genus Claviceps. Medicinal and Aromatic Plants - Industrial Profiles . CRC Press, 2004, ISBN 0203304195 , pp. 202-230.
- ^ Cohen ML, Kurz KD, Mason NR, Fuller RW, Marzoni GP, Garbrecht WL: Pharmacological activity of the isomers of LY53857, potent and selective 5-HT2 receptor antagonists . In: J Pharmacol Exp Ther . 235, No. 2, 1985, pp. 319-323. PMID 4057073 .
- ^ Cohen ML, Fuller RW, Kurz KD, Parli CJ, Mason NR, Meyers DB, Smallwood JK, Toomey RE: Preclinical pharmacology of a new serotonergic receptor antagonist, LY281067 . In: J Pharmacol Exp Ther . 244, No. 1, 1988, pp. 106-112. PMID 3335993 .
- ↑ Kao HT, Adham N, Olsen MA, Weinshank RL, Branchek TA, Hartig PR: Site-directed mutagenesis of a single residue changes the binding properties of the serotonin 5-HT2 receptor from a human to a rat pharmacology . In: FEBS Lett . 307, No. 3, 1992, pp. 324-328. PMID 1644189 .
- ↑ Johnson MP, Loncharich RJ, Baez M, Nelson DL: Species variations in transmembrane region V of the 5-hydroxytryptamine type 2A receptor alter the structure-activity relationship of certain ergolines and tryptamines . In: Mol Pharmacol . 45, No. 2, 1994, pp. 277-286. PMID 8114677 .
- ↑ a b Cole RJ, Kirksey JW, Dorner JW, Wilson DM, Johnson J Jr, Bedell D, Springer JP, Chexal KK, Clardy J, Cox RH: Mycotoxins produced by Aspergillus fumigatus isolated from silage . In: Ann Nutr Aliment . 31, No. 4-6, 1977, pp. 685-691. PMID 350117 .
- ↑ Lieberman AN, Gopinathan G, Estey E, Kupersmith M, Goodgold A, Goldstein M: Lergotrile in Parkinson disease: further studies . In: Neurology . 29, No. 2, 1979, pp. 267-272. PMID 34808 .
- ↑ Crider AM, Robinson JM, Floss HG, Cassady JM, Clemens JA: Ergot alkaloids. Synthesis of 6-alkyl-8-ergolenes and 6-methyl-8-aminoergolines as potential prolactin inhibitors . In: J Med Chem . 20, No. 11, 1977, pp. 1473-1477. PMID 915908 .
- ↑ Pinheiro EA, Carvalho JM, Dos Santos DC, Feitosa Ade O, Marinho PS, Guilhon GM, de Souza AD, da Silva FM, Marinho AM: Antibacterial activity of alkaloids produced by endophytic fungus Aspergillus sp. EJC08 isolated from medical plant Bauhinia guianensis . In: Natural Product Research . 27, No. 18, 2013, pp. 1633-1638. doi : 10.1080 / 14786419.2012.750316 . PMID 23234304 .
- ^ Timms GH, Tupper DE, Morgan SE: Synthesis of novel 8- and 10-substituted clavine derivatives . In: J. Chem. Soc., Perkin Trans . 1, 1989, pp. 817-822. doi : 10.1039 / P19890000817 .
- ↑ Bach NJ et al. (1980): J. Med. Chem., P. 492.
- ↑ Mategani S (2001): J. Heterocycl. Chem., P. 759.
- ↑ Rastogi S. (1970): Indian J. Chem., P. 377.
- ↑ Horwell DC, Tupper DE, Hunter WH (1983): J. Chem. Soc. Perkin Trans. I, p. 1545.
- ^ Hunter WH, Tupper DE (1987): J. Chem. Soc. Perkin Trans. I, p. 707.
- ↑ Stamos IK et al. (1995): J. Heterocycl. Chem., P. 1303.
- ↑ Tscherter H, Hauth H: Three new ergot alkaloids from saprophytic cultures of Claviceps paspali Stevens et Hall. 77. Communication on ergot alkaloids . In: Helvetica Chimica Acta . 57, No. 1, 1974, pp. 113-121. doi : 10.1002 / hlca.19740570111 .
- ↑ Inzce M et al. (2008): Tetrahedron, p. 2924.
- ^ Hunter WH (1986): Brit. UK Pat. Appl., GB 2162182 A 19860129.
- ↑ Mantegani S, Brambilla E, Varasi M: Ergoline derivatives: receptor affinity and selectivity . In: Farmaco . 30, No. 5, 1999, pp. 288-296. PMID 10418123 .
