Von Braun dismantling
The Von Braun degradation (also known as the Von Braun reaction or Von Braun amide degradation ) is a name reaction in organic chemistry . The reaction was named after its discoverer, the German chemist Julius von Braun (1875–1939). This synthesis produces haloalkanes and nitriles .
Overview reaction
In this degradation reaction, a secondary (or tertiary ) carboxamide with halogenated phosphorus ( halogenated either with bromine or chlorine) is converted into a nitrile and a haloalkane :
The radical R 1 can be an aryl radical (for example a phenyl radical) or a tertiary alkyl group , the radical R 2 is an alkyl group and the radical R 3 can be a hydrogen atom , an alkyl radical or a benzyl radical .
Reaction mechanism
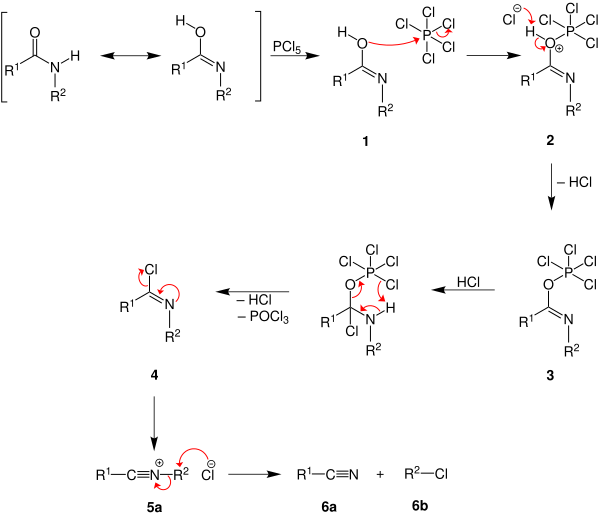
The possible reaction mechanism is taken from the book " Comprehensive Organic Name Reactions and Reagents " and is shown using phosphorus pentachloride as an example . To simplify the overview, the reaction mechanism is presented in two steps:
The carboxamide 1 reacts in the enolized form with the phosphorus pentachloride , with the elimination of a chloride ion , the oxonium ion 2 is formed . The chloride ion can now deprotonate the oxonium ion with the formation of hydrogen chloride. The resulting imine 3 then reacts with the hydrogen chloride to form an amine. As a result, the nitrogen is protonated, which means that it is deprotonated again by the phosphorus chloride residue and hydrogen chloride is split off. In addition, phosphorus oxychloride is also split off and the imine 4 is formed . By splitting off a chloride ion, a nitrile ion 5a is formed that can now react further in two different ways. Possibility one is the halogenation of the radical R 2 with elimination of a nitrile 6a and a haloalkane 6b .
Since alkenes are also formed as a by-product in the reaction, this could be explained by the following reaction mechanism:
With the elimination of nitrile, a carbenium ion is formed . There are now two further possibilities, either the chlorine ion combines with the carbenium ion to form a haloalkane 6b or the alkene 6c is formed by splitting off a proton .
Individual evidence
- ↑ von Braun, J .: About 1.5-Dibromopentane . In: Reports of the German Chemical Society . tape 37 , no. 3 , 1904, pp. 3210-3213 , doi : 10.1002 / cber.190403703118 .
- ↑ Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 2900.
- ↑ a b Z. Wang (Ed.): Comprehensive Organic Name Reactions and Reagents, 3 Volume Set . John Wiley & Sons, Hoboken, New Jersey 2009, ISBN 978-0-471-70450-8 , p. 2901.
- ↑ M. Windholz (Ed.): The Merck Index, Ninth Edition . Merck & Co., 1976, ISBN 978-0-911910-26-1 , p. ON-95.