Sodium periodate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
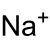 
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium periodate | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | NaIO 4 | |||||||||||||||
| Brief description |
colorless and odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 213.89 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.87 g cm −3 |
|||||||||||||||
| Melting point |
From about 300 ° C (decomposition) |
|||||||||||||||
| solubility |
moderate in water (91 g l −1 at 20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium periodate is a chemical compound from the group of periodates (more precisely the sodium salt of metaperiodic acid ). It is in the form of a very reactive, oxidizing, odorless and colorless powder.
Occurrence
Sodium periodate occurs naturally in small amounts as an admixture of Chile's nitrate .
Extraction and presentation
Sodium periodate can be represented from sodium iodate .
properties
When heated above 300 ° C, sodium periodate decomposes, producing sodium oxide and iodine (or hydrogen iodide in the presence of moisture ). Sodium periodate crystallizes tetragonally , space group I 4 1 / a (space group no. 88) , with the lattice parameters a = 5.337 Å and c = 11.95 Å.
use
Sodium periodate is used as a standard solution . It can also be used for the production of 1-pyrroline and as an oxidizing agent (e.g. as a cooxidant in dihydroxylation ). In bioorganic chemistry it is used for the selective oxidation of amino-terminal serines in peptides or proteins. In this way glyoxal functions are generated. These aldehydic groups can be used for bioconjugation with primary amines in Schiff base reactions. Aldehyde functions can also be generated from 1,2-diols, which occur in sugar residues of glycated biomolecules, by means of oxidation mediated by sodium periodate.
safety instructions
Sodium periodate itself does not burn, but it can promote existing fires considerably and reacts violently with combustible substances, so that contact with such can lead to spontaneous combustion.
See also
Individual evidence
- ↑ a b c d e f Entry on sodium periodate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 .
- ^ A. Kalman, DWJ Cruickshank: Refinement of the Structure of NaIO 4 . In: Acta Crystallographica , B26, 1970, pp. 1782-1785, doi: 10.1107 / S0567740870004880 .





