Watering can mold
| Watering can mold | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
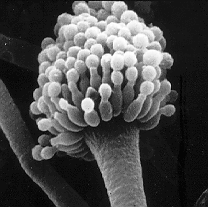
Electron microscope image of the Aspergillus fumigatus spore carrier |
||||||||||||
| Systematics | ||||||||||||
|
||||||||||||
| Scientific name | ||||||||||||
| Aspergillus | ||||||||||||
| P. Micheli ex Link |
The watering can mold ( Aspergillus ) are a genus of molds comprising over 350 species with aspergillus- shaped spore carriers . They are saprobionts that are widespread all over the world and live predominantly in dead, decomposing organic matter and make up a significant part of the material cycle in the earth's ecosystem . However, some species are pathogens that can affect humans, various animals or plants.
The genus Aspergillus has long been counted among the Fungi imperfecti , which differ from other fungi in that they only reproduce asexually . It is now known that some species go through a pleomorphic development cycle in which an asexual form ( anamorphic ) and a sexual form ( teleomorphic ) alternate. So far, the asexual forms will continue as provisional Aspergillus referred, while the known sexual forms under different generic names to the sac fungi be expected (Ascomycota).
The name Aspergillus goes back to the Italian priest and botanist Pier Antonio Micheli , whom the shape of the mushroom reminded of an Aspergillus (holy water sprinkler).
description


Watering can molds form whitish, greenish, black, red, brown, grayish or yellow fungal lawns that grow in so-called colonies . These colonies initially consist of a dense network of hyphae called the mycelium . The hyphae measure between 3 and 5 micrometers and are very variable in length. In A. nidulans , they are between 110 and 160 micrometers long and divided into 30 to 60 micrometers long compartments. However, this can vary considerably with other species, mutants or changed environmental conditions. Mycelium growth is exponential at first , but then gradually slows down. With increasing growth, the hyphae branch out at their tips and the typical widely branched mycelium develops. With age, the colonies appear increasingly and furrows form. This can lead to areas with anaerobic metabolism or areas in which nutrients are no longer available within the colony .
During fructification , aspergillus-like conidia carriers are formed, which are used for reproduction and on which conidiospores ( conidia ) mature. The conidiophores consist of foot cell , conidiophore , vesicle and phialides . The term conidiophore is used inconsistently, however, and is occasionally used synonymously with the entire conidium carrier.
Foot cells
The first sign of conidiogenesis is the swelling of cells within the mycelium, which are then called the conidogenic locus. These cells then form a septum (a strong thickening of the cell wall ). From each of these so-called foot cells , a single conidiophore grows as a branch perpendicular to the longitudinal axis of the cell and usually approximately in its center. As the conidium carrier continues to grow, the foot cell bends and twists increasingly. Their connection to the vegetative hyphae becomes more and more inconspicuous. Foot cells can develop both within the substrate and on aerial hyphae. The presence of foot cells is a very clear feature of the Aspergillus genus , but they are also found in a few other genera such as Sterigmatocystis .
Conidiophore
The structure that grows vertically out of the foot cell is called a conidiophore or simply a stalk. It forms the conidial head. In almost all types of conidiophore is unbranched, with a few exceptions, however, can be found in the sections Aspergillus , Cervini , Sparsi and Cremei . The cell wall of the conidiophore is uniform or increasingly thickened towards the base. The stem is often unseptate (without internal partitions), but in some species it can also be septate. In this case the septa are poorly developed. The outer wall of the conidiophore is smooth or rough, the inner wall often shows irregular thickenings. If a conidiophore breaks, the break edge usually resembles broken glass. The color varies between species and ranges from green to yellow to brown.
Vesicle
As conidiogenesis progresses, the conidiophore thickens at its tip and forms a spherical, hemispherical, ellipsoidal or elongated club-shaped vesicle (also called a bubble ). In some species from the Cervini , Restricti and Fumigati sections , the vesicle is not perpendicular to the conidiophore, but is kinked; in some of these species the vesicle is also fork-shaped. The color of the vesicle is often more intense than that of the stalk.
Phialides
On vesicles conical outgrowths grow phialids called. These are mostly hyaline or the same color as the bladder. Often they do not cover the entire surface of the vesicle, but only grow on certain fertile surfaces. Your cells have either very thin or stable cell walls that leave impressed traces in the vesicle when the phialides fall off at the end of conidiogenesis.
The phialides are either in one layer or in two layers one above the other. In the latter case, two or more secondary phialides grow out of each primary phialidus, called prophilalidus. These sit crown-shaped fork-shaped or whorled at the top of Prophilalidus. A phialidus is more or less cylindrical at the base and then narrows conically to a point at which there is a narrow conidia-producing tube.
In almost all species the vesicle contains only one nucleus. In the phialids, several cell nuclei arise through nucleus division, which, if present, are then passed on to the secondary philalids. A. brunneus is an exception , where the cell nuclei are passed on from the vesicle to the phialides. The cell nuclei are then pushed into the conidia-producing tube and tied off there one after the other. As a result, a single, unbranched chain of conidia arises from each tube.
Conidia
In almost all Aspergillus species, each conidia contains only one nucleus. However, some species of the Aspergillus section produce conidia with up to 12 cell nuclei. At least in A. brunneus and A. reptans these additional cell nuclei arise from continued division in the young conidia. This is unclear for other species. Even the conidia that have already been constricted are still supplied with nutrients by the vesicle until they have fully matured. The color of the ripe conidia varies from olive-brown, red-brown to light-green or almost yellow, depending on the species. The individual conidia are connected by very fine bridges within the chain.
distribution
Watering can molds are cosmopolitan . A meta-analysis from 2002 that evaluated 250 other studies came to the conclusion that most species prefer to live between the 26th and 35th parallel, i.e. in the subtropics . The Aspergillus section seems to have specialized primarily in deserts . The Ornati section, on the other hand, has its distribution focus closer to the temperate zones up to the 45th parallel. In general, watering can molds prefer tropical climates, whereas brush molds are more common in temperate latitudes.
Still, watering can mold is not limited to warm areas. Several Aspergillus species have been isolated in permafrost soil and ice samples from the Antarctic . Many species are also extremely salt-tolerant and can handle osmotic stress well (compare halophiles ). A. sydowii even lives as a pathogen on Caribbean horn corals in the sea.
Aspergillus conidia are part of the aerosol in the air. A long-term study from 1963 to 1991 in Cardiff measured an average conidia concentration between 45 and 110 spores per cubic meter of air. The measured maximum concentration was over 100,000 spores in one cubic meter. The concentration is lower in summer than in winter.
The spores can be blown very high. One study found A. calyptratus spores at an altitude of 4,100 meters. A. niger spores were found up to 3,200 meters, A. glaucus conidia up to 2,200 meters and the spores of A. fumigatus and A. flavus at an altitude of 1,400 meters. The wind can blow the spores very far away, for example spores were carried from the Sahara to the Caribbean.
Life cycle and ecology

The life of a watering can mold usually begins as a conidia . However, an ascospore from an assigned teleomorph is also rarely the origin of an Aspergillus . When the conidia hit a solid or liquid surface, they settle there and start to germinate depending on temperature, humidity, pH and other conditions. The conidia initially swells and a germinal thread grows out. The situation is analogous for the ascospores. Cell division creates several interlinked elongated cells that represent a hypha . As the hyphae grow, they branch out. A hyphae in its entirety is called the mycelium . If growth has progressed far enough and sufficient nutrients are available, fructification begins and the spore carriers develop, on which conidia mature asexually.
Many Aspergilli are known to have a pleomorphic development cycle, which means that they have a sexual form (main fruit form, teleomorph) and an asexual form (secondary fruit form, anamorphic ). Many species are so-called Fungi imperfecti , which means that it is unknown whether they reproduce exclusively asexually or whether the phase of sexual reproduction has not yet been discovered. The name of a pleomorphic mushroom species with all its fructification forms, anamorphic and teleomorphic, is holomorphic . For the holomorphs, the name of the teleomorph is usually used.
Under certain environmental conditions, some Aspergillus species begin to reproduce sexually. They form cleistothecia (almost round Ascomata ) in which ascospores develop. Strictly speaking, these Cleisthotecia are no longer organs of an Aspergillus , since the species now bears the name of the teleomorph. Since the anamorphic genus Aspergillus belongs to different teleomorphic genera, the shape of these sexual organs is very different. For example, in A. alliaceus, the cleistothecium of the teleomophan Petromyces alliaceus is embedded in a thick-walled sclerotium . In the genus Eurotium , which is the teleomophe genus belonging to the Aspergillus section, the cleistothecia are small, bare, yellow and sit on stem-like hyphae. In the genus section Emericella (teleomorphs to section Nidulantes ) they are dark purple and surrounded by thick-walled envelope cells .
The question of whether all Aspergilli have teleomorphs is unclear and controversial. For example, A. fumigatus was one of the best-researched watering can molds, but over 100 years of research had not succeeded in isolating the main fruit form. Many researchers took this as an indication that only the minor crop form exists. When Celine M. O'Gorman, Hubert T. Fuller and Paul S. Dyer discovered the teleomorph Neosartorya fumigata in 2009 , this argument was put into perspective . However, this does not clarify whether all Aspergilli are really capable of sexual reproduction.
nutrition
One of the most salient characteristics of the mushroom kingdom is the way in which it absorbs nutrients. They secrete acids and enzymes into their environment, which break down the macromolecules present there into simpler compounds that can then be absorbed by the fungi. Put simply, this means that mushrooms first digest their food and then ingest it. Watering can mold grows through potential food with their hyphae and then increasingly decomposes them. Typical names for this process are mold and rot .
Many human foods are also attractive to Aspergillus species. Difficult habitats are also colonized. For example, Aspergilli from the Aspergillus section were isolated from salted dried fish . Another, as yet unidentified, watering can mold is even able to grow on low - carbon coal .
Natural enemies
A wide variety of insects, especially beetles (Coleoptera), eat mushrooms. Some species have specialized in watering can mold as food. On the other hand, many secondary metabolites, especially aflatoxins produced by Aspergilli, are highly toxic to insects.
The tropical mold beetle ( Ahasverus advena ) eats the conidial heads of A. glaucus , but avoids the Ascomata of the teleomorphs. The gloss beetle (Nitidulidae) Carpophilus freemani has specialized entirely in Aspergillus and hardly eats anything else. The inguinal flat beetle ( Cryptolestes ferrugineus ) eats A. fumigatus , A. niger , A. versicolor and A. ochraceus , but disdains A. flavus , which produces many aflatoxins. The baked fruit beetle Carpophilus hemipterus, on the other hand, has specialized in these very poisonous Aspergilli. He is immune to 25 ppm aflatoxins.
Mycoviruses
One of the very first mycoviruses was discovered in A. foetidus in 1970 . It was a double-stranded RNA virus . In the same year, the transmission of virus-like particles in A. niger was also described.
Mycoviruses can affect their hosts. It has been observed that infected A. niger and A. tubingensis colonies suffer from greatly reduced hyphae growth and spore production decreases. Infected individuals from the Flavi section or infected A. nidulans colonies, however, had little effect.
Mycoviruses have now been detected in more than 25 different species of Aspergilli, with twelve different viruses from nine families occurring.
Pathogenicity
The ability of watering can mold to live on many different substrates under a wide range of environmental conditions means that some species can also overgrow living or dead tissues of humans or animals. The infestation of living tissue is the cause of various diseases. However, such an infestation is always accidental, since all Aspergillus species are actually saprobionts. In addition to the direct infestation of tissue, many Aspergillus species produce toxic or allergenic secondary metabolites .
Historical
In 1748, William Arderon discovered a fungus that grew on a live fish. Geoffrey Clough Ainsworth calls this the first record of a fungus as a pathogen on a vertebrate. In 1815, Réaumur found an unknown mold in the air sac of a mountain duck ( Aythya marila ). Franz Unger discovered the first pathogenic fungus in a person in 1833 when he was studying thrush . However, he considered the fungus to be an effect of the disease and not its cause.
In 1842, Rayer and Montagne discovered an A. candidus colony in the air sac of a bullfinch ( Pyrrhula pyrrhula ). However, images of a mushroom from the air sac of the same species by Deslongchamps from 1841 (one year earlier) suggest that it is an Aspergillus species. Robin discovered A. fumigatus in the air sac of a pheasant ( Phasianus colchicus ) in 1852 .
The first report of aspergillosis in mammals probably dates back to 1841, when Rousseau and Serrurier found fungi in the lungs of an axis deer ( axis axis ). Unfortunately, the description of the fungus is short and imprecise. Aspergillosis as a human disease was first described by Rudolf Virchow in 1856. These aspergillosis were probably caused by A. fumigatus .
Diseases caused by A. niger were first described by Cramer in 1859, diseases caused by A. nidulans and A. flavus by Siedemann in 1889. A. terreus was identified as the pathogen in 1922 by Langeron .
Infections
Aspergillus infections often develop in the lungs of mammals or birds. The most important pathogen in the genus is Aspergillus fumigatus . An aspergilloma can form as an acute disease in healthy organisms . This is a globular colony in the lungs or sinuses , similar to a ball of fungus that lodges in the organ. People are particularly at risk if there are lung caverns or scarred tissue from previous diseases, such as after tuberculosis . Aspergillomas are common in farm animals.
If inhaled Aspergillus spores grow in the lungs and are not restricted to a compact colony, acute aspergillosis develops . Hyphae form in the lungs and then mycelium, which eventually spreads throughout the body through the bloodstream. Metastases form on the organs and in the central nervous system . Acute aspergillosis does not occur in adults with an intact immune system; it is very rare in children. It is not uncommon for immunosuppressed patients, for example after a bone marrow or stem cell transplant or AIDS patients, to develop it. Invasive aspergillosis is a dangerous infection with a high lethality in the range between 50% and 95%. Birds in particular often develop acute aspergillosis. In chicks of domestic fowl , the disease is Aspergillus pneumonia called and often leads to mass mortality in farms. Epidemics also occur repeatedly in wild birds and have been observed in African ostriches ( Struthio camelus ) and herring gulls ( Larus argentatus ) , among others . In parrots (Psittaciformes) lung and air sac mycoses caused by Aspergillus occur . Among mammals, diseases are common in lambs , but very rare in domestic cattle calves. Epidemics occurred in rabbits and guinea pigs (Caviidae). For horses ( Equus ) are especially Luftsackmykosen feared. These are mostly caused by A. fumigatus , but also by other Aspergilli or fungi of the genera Penicillium or Mucor .
In addition to acute aspergillosis, a chronic form of the disease can also develop. This either follows an overcame acute course of the disease or develops slowly without initially showing symptoms. Chronic aspergillosis occurs sporadically in horses, sheep ( Ovis ) or monkeys . Such chronic forms are common in birds and particularly common in waterfowl . In penguins (Spheniscidae) this disease has a considerable influence on the breeding success.
There is no evidence that Aspergillus species can grow on keratin . For this reason, they are out of the question as skin fungi . Subcutaneous colonies of A. glaucus have rarely been found in domestic chickens . Diseases of the ear are more important . Aspergillus species can grow on wax , epithelial deposits, or exudate and then damage the inner ear. Eye diseases are also not uncommon. Aspergillus species can cause keratitis , an inflammation of the cornea . Such diseases are particularly common in chickens.
Infections in the genital tract also occur. In cattle and horses, miscarriages can result from Aspergillus infections in the genital area. Only one human case was known from 1959. In birds, Aspergillus species attack the eggs and break them down. With chicken eggs, the embryo usually dies after the sixth day after the egg is infected.
Some Aspergillus species can live as facultative endoparasites in insects and cause real animal diseases . The fungi penetrate the insects and feed on the hemolymph . A. flavus, for example, attacks butterfly species such as the European corn borer ( Ostrinia nubilalis ) or Hyalophora cecropia , but has also been detected in jumping horror (Orthoptera), which in turn are regularly attacked by A. parasiticus . Economic damage is mainly caused by A. fumigatus and A. ochraceus , which infest honey bees ( Apis ). The infestation of silk spiders ( Bombyx mori ) by various Aspergillus species also repeatedly causes economic damage.
Various antimicrobial drugs (antimycotics) are used to treat infections with Aspergillus species (including voriconazole, caspofungin, posaconazole or itraconazole).
Allergies
One of the most important aspects of the pathogenicity of Aspergilli is that almost all species produce allergens . As a consequence, inhaling spores can trigger allergic reactions .
Allergies caused by Aspergillus almost exclusively affect the respiratory system , but mild skin reactions are also very rarely reported. A mild form is called Aspergillus asthma . More severe forms are allergic bronchopulmonary aspergillosis , in which the lungs are heavily colonized by eosinophilic granulocytes .
A chronic form is the so-called farmer's lung , which can lead to scarring of the lung tissue ( pulmonary fibrosis ).
Toxicoses
Aspergillus species produce both endotoxins and exotoxins . These are of great importance for humans and animals because the saprobiontic fungi can also thrive on food and thus enter the organism. Such poisonings are called aspergillus toxicoses or aspergillotoxicoses.
Species from the Clavati section mainly produce patulin . This poison is often found as an impurity in moldy fruits, vegetables, grains and other foods, as well as in moldy corn silage; However, the most important sources of contamination are apples and apple products, often apple juice. Since patulin can also appear in fruits that are not visibly damaged or spoiled on the outside, the contamination cannot be completely eliminated by removing all visibly damaged or spoiled fruits. The lethal dose LD 50 is 25 to 35 milligrams of spores per kilogram of body weight in mice. Spores from the Nigri section are only slightly poisonous, they contain large amounts of oxalic acid . Fungi from the Aspergillus section are also only slightly poisonous.
The mushrooms from the Fumigati section are more important , they mainly produce three toxins: fumigatin , helvolic acid and gliotoxin . The lethal dose LD 50 in mice is 1.5 milligrams per kilogram of body weight.
Species from the Flavi section are even more poisonous ; they produce a large number of aflatoxins , especially dehydrofurans . In dogs the lethal dose LD 50 is 200 micrograms per kilogram of body weight, in birds it is significantly lower. The high toxicity of A. flavus gave rise to the theory in the 1980s that the species was used by the ancient Egyptians to protect their graves and that it was responsible for the so-called curse of the Pharaoh .
A. flavus poisons are deadly to almost all insects, but fungus species that do not contain aflatoxins can also be dangerous to insects. For example, kojic acid , which is produced by many species, but especially by A. flavus , is poisonous for the silkworm ( Bombyx mori ). A. parasiticus kills the mealybug (Pseudococcidae) Saccharicoccus sacchari regardless of whether it is the wild type or a mutant that cannot produce aflatoxins.
The mycotoxins from the Clavati , Fumigati and Flavi sections are, in addition to their direct harmful effects, highly carcinogenic (carcinogenic).
Aspergillus as a plant pathogen
In addition to animals and humans, Aspergilli can also damage plants as phytopathogens . In his work from 2004, János Varga lists a total of 30 important plant diseases caused by Aspergillus species and over 50 host plants. Some important diseases are, for example, chlorosis in almonds ( Prunus dulcis ), which is caused by A. niger , or albinism in citrus plants ( Citrus ), caused by A. flavus .
The black rot of the onion and the peanut crown rot , which regularly cause great economic damage, are infections by A. niger . The dreaded vine cancer in viticulture is also triggered by the same species. A. fischerianus, on the other hand, preferentially attacks cranesbills ( Geranium ), whereas A. aculeatus , for example, also inhabits grapevines ( Vitis vinifera ).
A large number of Aspergilli attack coffee plants ( Coffea ). Many mycotoxins also find their way into human food in this way . A. flavus regularly causes great damage, especially in cotton production .
genetics

Guido Pontecorvo became interested in the genetics of A. nidulans around 1950 . In 1952 he first described the parasexual reproduction of the species and recognized the mechanism by which two haploid cell nuclei spontaneously fuse to form a mitotic diploid . If the cell nuclei have a different genetic constitution, the nucleus formed is heterozygous for certain genes . In the diploid phase, homologous chromosomes can now be recombined . Thereafter, through the gradual loss of chromosomes in the course of a series of cell divisions, haploid nuclei are formed again. In this way, anamorphs can adapt to changed environmental conditions without the possibility of sexuality.
Although parasexuality was discovered in a species to which a teleomorph ( Emericella nidulans ) with sexuality in the narrower sense also exists, the transformation quickly became an alternative to crossbreeding in the Aspergillus genetics. Long before recombinant DNA became available, genetic markers were recombined by exploiting parasexuality . A. nidulans quickly became the most important eukaryotic model organism of all.
The first genetic work on the cell cycle was also carried out on A. nidulans . The knowledge about catabolite repression , nitrogen catabolite repression , pH regulation , polar growth , signal transduction and the morphogenesis of mycelium-forming microorganisms were fundamentally advanced through work on the model organism A. nidulans . For example, γ-tubulin on an A. nidulans mutant was discovered as an important result .
In 2003, the complete is the DNA sequence of the genome of A. nidulans published. In December 2005, the sequences of A. fumigatus , A. flavus and A. oryzae (= A. flavus var. Oryzae ) were published together in a single issue of Nature . This simultaneous publication of three genomes quickly made Aspergillus the most important genus for comparative genomics of fungi. There was also a strong heterogeneity within the genus. The following year the complete sequence of A. niger followed . The smallest Aspergillus genome sequenced to date is that of A. fumigatus with 29.3 megabase pairs (Mb), the largest that of A. flavus var. Oryzae with 37.1 Mb. A. fumigatus has 9,926 genes , whereas A. flavus var. oryzae has 12,071 genes. The size of the A. niger genome is 33.9 Mb between the other two species.
Fully genomic microarrays now exist for A. nidulans , A. fumigatus , A. flavus and A. oryzae . So far, studies have mainly been carried out on the optimization of fermenters and on various secondary metabolites.
Systematics
The name watering can mold comes from the shape of the conidium carriers. Under the microscope, these look similar to the shower head of a watering can or a feather duster. The scientific name Aspergillus is also derived from the shape of the conidium carriers.
Mycological history


Various molds were constantly present in the human environment. Before the development of the light microscope around 1600, the description was limited to the colors of the colonies. Pier Antonio Micheli was the first to examine spores and spore carriers under a microscope in 1729. He observed that the spore chains extended radially from a central axis. The structure reminded him of an aspergillus , a liturgical device that is used to sprinkle holy water . That is why he used the name Aspergillus for the mold he observed.
Micheli took the term Aspergillus very broadly and described almost all of the mold he observed as Aspergillus . This very broad conception of the genre lasted a long time. For example, Albrecht von Haller described several Aspergillus species in the 18th century , which were later assigned to other genera, such as Sporodina . Christian Hendrik Persoon, on the other hand, rejected the genus Aspergillus in its entirety in his works from 1797 and 1801 and added it to the very broad genus Monilia described by him , as he understood the spore chains as a string of holoblastic Monilia conidia.
Heinrich Friedrich Link, in turn, rejected this view of Persoon, as he was of the opinion that the shape of the spore chains results directly from the presence of a conidiophore. However, Link's description of the conidia carriers is very imprecise, which was probably due to the poor herbarium evidence that was available to him. The first precise description of the conidiophore and the enteroblastic conidia was found around 1828 by August Karl Joseph Corda . His descriptions were very precise, but complicated and incomprehensible, so that by 1850 there were probably only a handful of people who could identify Aspergillus species. In 1856 Montagne complained that every white horse he looked at was a new species.
In 1854 Anton de Bary discovered that the conidia of Aspergillus glaucus and the cleistothecia of the species Eurotium herbariorum described by Link arise from the same mycelium. This finding was confirmed by Fresenius , Cramer and Oscar Brefeld .
Victor Félix Raulin and van Tieghem began in France in 1860 with the production of gallic acid by fermenting vegetable gall and studied the mold involved. In 1901 Carl Friedrich Wilhelm Wehmer published the first comprehensive monograph on some new Aspergillus species . The Aspergilli by Charles Thom and Margaret Brooks Church appeared in 1926 , in this work the genre was first divided into groups. The monograph describes 350 species in eleven groups.
In the meantime a lot of smaller articles about the genre had appeared and the situation was very confusing. Hiroshi Tamiya and Tatsuyoshi Morita cited 2,424 individual articles in their work Bibliography by Aspergillus, 1729 to 1928 . Of these, only 115 were older than de Bary's essay from 1854, 309 articles appeared by 1891 and the remaining 2000 between 1891 and 1928.
System und Phylogenie by Adalbert von Blochwitz appeared in 1929 . He suggests a completely different division of the groups than Thom and Church. George Smith made systematic photomicrographs of many species for the first time in 1938 . In the 1940s, research on the Aspergillus genus was intensified again. Some species have already been used industrially to produce various organic acids, and the pathogenic potential of fungi has also attracted increasing interest among scientists. In 1945, Charles Thom and Kenneth B. Raper published A Manual of the Aspergilli . The monograph contained descriptions of 80 species, ten varieties and 14 groups. Kenneth B. Raper then published the book The Genus Aspergillus together with Dorothy I. Fennel in 1965 , which contained 132 species in 18 groups.
The internal systematics of the genus developed further when Robert A. Samson and John I. Pitt published the work Advances in Penicillium and Aspergillus Systematics in 1985 . They were dissatisfied with the taxonomically undefined term of the groups that Thom and Church had introduced and divided the genus into six subgenera and 16 sections. They deepened this view again in their work Modern Concepts in Penicillium and Aspergillus Classification , which the two published in 2000.
In 2002 a kind of identification key for watering can mold appeared, albeit with molecular biological markers , under the title Identification of Common Aspergillus Species . In 2008, Samson and János Varga published the work Aspergillus Systematics in the Genomic Era , in which they revised the taxonomic classification. Finally, the work Aspergillus: Molecular Biology and Genomics , edited by Masayuki Machida and Katsuya Gomi , appeared in February 2010 , in which it is more strongly pointed out that the Aspergilli are not a classical genus, but a form taxon . Nevertheless, the division of Samson and Pitt into subgenera and sections is retained in this work.
External system
If Aspergillus is viewed as a form taxon, the watering can mold are also not viewed as a family group; in this reading they are rather a common type of organization.
If, however, Aspergillus is considered a genus , it is part of the Trichocomaceae family . David Malloch divided this family into two subfamilies in 1985 , the Trichocomiideae with the anamorphic genera Penicillium and Paecilomyces and the Dichlaenoideae with the anamorphic Aspergillus , Penicillium , Merimbla , Paecilomyces and Polypaecilum . The fact that the same anamorphs occur in both subfamilies already shows that these are not monophyletic groups.
A study from 1995, which looked at ribosomal DNA in addition to morphological features , revealed the genera Monascus and Eupenicillium as the closest relatives of the watering can mold. The study also suggests that the Aspergilli may be a monophyletic group. Three years later, a Japanese research group investigated the phylogeny using the 18S rRNA . As a result, the anamorphic genera Penicillium and Geosmithia were identified as the closest relatives of the Aspergilli. This study also suggests the monophyly of the group Aspergillus in the family of Trichocomaceae sensu Malloch and Cain 1972. A more recent molecular genetic study from 2000 comes to the conclusion that the watering can mold is not a monophyletic group. Nevertheless, the question has not yet been finally clarified (as of March 2010).
nomenclature
The status of the Aspergilli is highly controversial. The main problem is that this group clearly belongs together morphologically, but that the sexual forms are divided into a total of eleven genera. The handling of the situation is not clearly clarified. The International Code of Botanical Nomenclature has regulated in 1910, in paragraph 59, that in this case two names may be awarded for a kind, one for the Anamorphic and one for the Teleomorphs. This rule is still valid today (as of 2010). In 2003, however, the proposal was made to discard this dual nomenclature and to assign only one name to a species, in which case the generic name of the teleomorph would be used. For many watering can mold, however, the teleomorph is unknown or even non-existent. In this case, it was proposed to transfer the species to the likely teleomorphic genus, even if the teleomorphic genus is actually unknown.
Another proposal is to keep the Aspergilli as a form taxon and to keep the name Aspergillus for those species for which the teleomorph is unknown for the time being.
Internal system
Stephen W. Peterson, Janos Várga, Jens C. Frisvad and Robert A. Samson divided the Aspergilli in 2008 into eight subgenera and 22 sections . This classification was made according to molecular genetic aspects, but corresponds well with the classification of Kenneth B. Raper and Dorothy I. Fennel according to morphological criteria from 1965. Some sections that were created after 1965 according to chemical aspects (secondary metabolites) also remained receive. Masayuki Machida and Katsuya Gomi adopted this classification in their monograph from 2010.
During this investigation, the relationships between the individual sections were also determined. They are shown in the following cladogram :
|
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|
The Aspergillus species are linked to teleomorphs from a total of eleven genera. These are:
- Chaetosartorya Subram.
- Emericella Berk.
- Eurotium Link
- Fennellia B.J. Wiley et EGSimmons
- Hemicarpenteles A.K.Sarbhoy et Elphick
- Hemisartorya J.N. Rai et HJChowdhery
- Neocarpenteles Udagawa et Uchiy.
- Neopetromyces Frisvad et Samson
- Petromyces Malloch et Cain
- Sclerocleista Subram.
- Warcupiella Subram.
Most Aspergillus sections map exactly to one teleomorphic genus, conversely the teleomorphic genera do not necessarily map to an Aspergillus section. An exception in the first sense is the Ornati section , in which the main fruit forms from the genera Sclerocleista and Hemicarpenteles can be found. The Hemicarpenteles are probably also Sclerocleista , which can be transferred to this genus.
A similar exception occurs in the Clavati section . Just as there are many watering can molds for which the anamorph is unknown, there are also a number of teleomorphs from the genera assigned to the Aspergillus for which the anamorphs are unknown. A special case is the species Dichotomomyces cejpii ; a molecular genetic investigation showed that the species is very closely related to the species in the Clavati section and should be assigned to it. The anamorphic to the species is so far unknown. The same applies to the species Penicilliopsis clavariaeformis and the section Zonati .
The Aspergillus group is very species-rich and has not yet been definitively researched. Many of the species are unsafe or controversial. One reason for this is that many species have no or unusable type specimens . Especially for the species that were described in the 18th and 19th centuries, no or insufficient type specimens were created, or these were later lost. The old first descriptions are often short and insufficient by today's standards, so that it can no longer be clearly clarified whether the status as an independent species rightly exists.
If, on the other hand, type specimens have been created, the spores are no longer capable of germination after such a long storage time, but are still intact, so that they can be evaluated molecularly. In this way some taxonomic questions could already be answered. In some cases it was also possible to define a neotype .
Molecular genetics had a very big influence on the system of watering can mold. Many relationships could be clarified, varieties were upgraded to species , or species were downgraded to varieties. The best known example is probably A. oryzae . The well-known species used in fermentation was downgraded to a variety A. flavus var. Oryzae after molecular genetic testing . Nevertheless, the name A. oryzae is still very common today.
Currently (as of March 2010) there are 355 valid Aspergillus species, 354 of which are recent . The fossil species A. collembolorum was discovered in 2005 enclosed in an amber . The type species of watering can mold is Aspergillus glaucus , the species and their assignment are:
| Species of the genus Aspergillus | |
|---|---|
| Anamorphic | Teleomorphs |
| Aspergillus subgenus | |
|
|
|
Eurotium herbariorum (FH Wiggers) Link |
|
Eurotium appendiculatum Blaser |
|
Eurotium athecium (Raper et Fennell) Arx |
|
Eurotium latericium Mont. |
|
Eurotium echinulatum Delacr. |
|
Eurotium carnoyi Malloch et Cain |
|
- |
|
Eurotium costiforme H.Z.Kong et ZTQi |
|
Eurotium cristatum (Raper et Fennel) Malloch et Cain |
|
Eurotium chevalieri L. Mangin |
|
- |
|
- |
|
- |
|
Eurotium pseudoglaucum (Blochwitz) Malloch et Cain |
|
Eurotium niveoglaucum (Thom et Raper) Malloch et Cain |
|
- |
|
- |
|
- |
|
Eurotium heterocaryoticum C.M.Chr., LCLópez et CRBenj. |
|
Eurotium intermedium Blaser |
|
Eurotium leucocarpum Hadlok et Stolk |
|
- |
|
Eurotium medium R. Meissn. |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
Eurotium carnoyi Malloch et Cain |
|
- |
|
- |
|
Eurotium parviverruculosum H.Z.Kong et ZTQi |
|
Eurotium proliferans G.Sm. |
|
- |
|
Eurotium repens de Bary |
|
Eurotium rubrum Jos.King |
|
- |
|
- |
|
Eurotium spiculosum Blaser |
|
- |
|
Eurotium subgriseum Peck |
|
Eurotium taklimakanense Abliz et Y.Horie |
|
Eurotium testaceocolorans Novobr |
|
Eurotium tonophilum Ohtsuki |
|
Eurotium tuberculatum Z.T.Qi et ZMSun |
|
- |
|
Eurotium amstelodami L. Mangin |
|
Eurotium xerophilum Samson et Mouch. |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
Eurotium halophilicum C.M.Chr., Papav. et CRBenj. |
|
- |
|
- |
| Subgenus Fumigati | |
|
|
|
Neosartorya fumigata O'Gorman, Fuller, and Dyer |
|
- |
|
Neosartorya aureola (Fennell et Raper) Malloch et Cain |
|
- |
|
Neosartorya coreana S.B. Hong, Frisvad et Samson |
|
- |
|
Neosartorya fennelliae Kwon-Chung et SJKim |
|
Neosartorya fischeri (Wehmer) Malloch et Cain |
| - | |
|
- |
|
- |
|
|
|
Neosartorya galapagensis Frisvad, Hong et Samson |
|
- |
|
- |
|
Neosartorya hiratsukae Udagawa, Tsub. et Y. Horie |
|
Neosartorya aurata (Warcup) Malloch et Cain |
|
Neosartorya indohii Y.Horie |
|
Neosartorya laciniosa S.B. Hong, Frisvad et Samson |
|
- |
|
- |
|
Neosartorya multiplicata Yaguchi, Someya et Udagawa |
|
Neosartorya glabra (Fennell et Raper) Kozakiewicz |
|
Neosartorya nishimurae Takada, Y.Horie et Abliz |
|
- |
|
Neosartorya stramenia (Novak et Raper) Malloch et Cain |
|
Neosartorya quadricincta (E. Yuill) Malloch et Cain |
|
Neosartorya spathulata Takada & Udagawa |
|
Neosartorya sublevispora Someya, Yaguchi et Udagawa |
|
- |
|
Neosartorya takakii Y.Horie, Abliz et K.Fukush. |
|
Neosartorya tatenoi Y.Horie, Miyaji, Koji Yokoyama, Udagawa et Camp.-Takagi |
|
Neosartorya pseudofischeri S.W. Peterson |
|
Neosartorya tsurutae Y. theory |
|
- |
|
Neosartorya udagawae Y.Horie, Miyaji et Nishim. |
|
- |
|
- |
|
- |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
- |
|
Neocarpenteles acanthosporum (Udagawa et Takada) Udagawa et Uchiyama |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
| Subgenus Ornati | |
|
|
|
Sclerocleista ornata (Raper, Fennell et Tresner) Subram. |
|
- |
|
Hemicarpenteles thaxteri (Subramanian) Arx |
|
Hemisartorya maritima Rai et Chowdheri |
| Subgenus Nidulantes | |
|
|
|
Emericella nidulans (Eidam) Vuillemin |
|
Emericella acristata (Fennell et Raper) Subramanian |
|
- |
|
- |
|
- |
|
Emericella bicolor M. Chr. Et States |
|
- |
|
Emericella corrugata Udagawa et Y.Horie |
|
- |
|
Emericella dentata (Sandhu et Sandhu) Horie |
|
Emericella discophora Samson, Zalar et Frisvad |
|
- |
|
Emericella falconensis Y. Horie, Miyaji, Nishim. et Udagawa |
|
Emericella filifera Zalar, Frisvad et Samson |
|
Emericella foeniculicola Udagawa |
|
Emericella foveolata Y.Horie |
|
Emericella fruticulosa (Raper et Fennel) Malloch et Cain |
|
Emericella parvathecia (Raper et Fennel) Malloch et Cain |
|
Emericella miyajii Y.Horie |
|
Emericella montenegroi Y. Horie, Miyaji et Nishim. |
|
- |
|
- |
|
Emericella navahoensis M.Chr. et States |
|
Emericella olivicola Frisvad, Zalar et Samson |
|
Emericella omanensis Y.Horie et Udagawa |
|
- |
|
Emericella purpurea Samson et Mouch. |
|
Emericella qinqixianii Y.Horie, Abliz et RYLi |
|
- |
|
Emericella rugulosa (Raper et Fennel) CRBenjamin |
|
Emericella spectabilis M.Chr. et Raper |
|
- |
|
Emericella stella-maris Zalar, Frisvad et Samson |
|
Emericella variecolor Berk. et Broome |
|
Emericella striata (Rai, Tewari et Mukerji) Malloch et Cain |
|
Emericella sublata Y.Horie |
|
- |
|
- |
|
Emericella quadrilineata (Thom et Raper) CRBenjamin |
|
- |
|
Emericella undulata H.Z.Kong et ZTQi |
|
Emericella unguis Malloch et Cain |
|
Emericella unguis Malloch et Cain |
|
- |
|
Emericella venezuelensis Frisvad et Samson |
|
- |
|
Emericella violacea (Fennel et Raper) Malloch et Cain |
|
|
|
- |
|
- |
|
|
|
- |
|
- |
|
|
|
- |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
Emericella heterothallica (Kwon et al) Malloch et Cain |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
- |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
- |
|
- |
| Subgenus Terrei | |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
Fennellia flavipes B.J. Wiley et EGSimmons |
|
- |
|
Fennellia nivea (BJWiley et EGSimmons) Samson |
|
- |
|
- |
| Subgenus Circumdati | |
|
- |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
Neopetromyces muricatus (Udagawa, Uchiyama et Kamiya) Frisvad et Samson |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
- |
|
- |
|
Petromyces albertensis J.P. Tewari |
|
Petromyces alliaceus Malloch et Cain |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
Petromyces parasiticus B.W. Horn, I. Carbone et JHRamirez-Prado |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
|
|
Chaetosartorya cremea (Kwon-Chung et Fennell) Subramanian |
|
- |
|
Chaetosartorya chrysella (Kwon-Chung & Fennell) Subramanian |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
Chaetosartorya stromatoides B.J.Wiley et EGSimmons |
|
- |
|
- |
| Subgenus Warcupi | |
|
|
|
Warcupiella spinulosa (Warcup) Subramanian |
|
|
|
- |
|
- |
| Subgenus Candidi | |
|
|
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
| without assignment | |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
|
- |
Industrial use
A. flavus var. Oryzae , A. sojae and other closely related species have been used in large parts of Asia for the fermentation of food for over 1,500 years .
In Japan the mushrooms are known under the name Kōji ( Japanese 麹 ). Miso or soy sauce , for example, is produced by fermenting soy using Kōji . The mycelium grows through the substrate and the Aspergillus species releases enzymes and organic acids through the cell walls. The enzymes partially break down the carbohydrates and proteins from the substrate and change the taste. Similar methods of fermentation with aspergilli are also widespread in other countries in the region. Various soy pastes in the People's Republic of China are called jiàng (酱). One example is the yellow soy paste huángjiàng (黄酱), which is mainly eaten in the Beijing area . Various pastes fermented in this way are common in Korea and are sold under the name jang (醬, Hangeul 장). Similar pastes are also consumed in Indonesia and Thailand; in Vietnam they are called Tương . Whole fermented soybeans are called tao-tjo , in Thailand . A fermented fish sauce from Thailand is called nam pla (น้ำปลา).
Another use of watering can molds is the fermentation of rice using A. oryzae for sake or using a white kōji Aspergillus kawachii for shōchū . Awamori (Japanese 泡 盛 ) is another spirit that is primarily made in Okinawa , but is common throughout Southeast Asia. A black kōji Aspergillus awamori is used to make it.
The Japanese scientist Jōkichi Takamine brought Kōji to the United States in the late 19th century . There he extracted enzymes from the fungus using alcohol and marketed the result under the name Takadiastase as a remedy for digestive complaints. In 1894 he had the process patented and thus received the first US patent on a microbiological enzyme.
In 1895, Albert Boidin in France developed a process for the production of alcohol by working with Aspergillus sp. inoculated grain boiled away. Further pioneers in the field of industrial use of Aspergillus were Leo Wallenstein and Otto Röhm , who were the first to isolate and technically utilize enzymes .
The world's three largest fermentation companies, DSM , Novozymes and Genecor-Danisco , state that Aspergillus are their main organisms. For DSM this is A. niger and for Novozymes A. flavus var. Oryzae .
Citric acid production
Carl Wehmer discovered in 1891 that A. niger produced not small amounts of oxalic acid when sugar was broken down ; some citric acid was also formed . This discovery was not particularly sensational at first, as oxalic acid could already be produced more cheaply. In 1917, James N. Currie refined the process and created the possibility of industrial production of citric acid from A. niger . Currie marketed his idea to Pfizer Inc., which developed the process further. Until then, citric acid was made from lemons ( Citrus × limon ) and Italy had an economic monopoly on the product. Today almost 100 percent of the citric acid used comes from A. niger , with a world production of 1.6 million tons per year (as of 2007) and is produced in large bioreactors .
Citric acid and its salts are used to preserve and acidify food, for example in beverages. It is used in particular in lemonades and iced teas and is approved in the European Union as a food additive with the number E 330. Citric acid is also used in cleaning agents, cosmetics and medicine.
Other secondary metabolites
Today over 100 different enzymes are industrially produced from Aspergilli. Important groups are amylases , catalases , cellulases , lipases , phytases and xylanases .
Important secondary metabolites from A. niger are further gluconic acid , which, as a food additive (E 574) as Metallbeizmittel and in medicine as ferrous gluconate in the treatment of iron deficiency is used, as well as itaconic acid , which as a comonomer for the synthesis of polyacrylates and rubber is used . Also, cyanocobalamin (vitamin B 12 ) is with the help of A. niger produced.
Kojic acid is obtained primarily with the help of A. flavus and is used in cosmetics for skin bleaching. In 1980, Merck patented lovastatin , a secondary metabolite of A. terreus that is now used to treat hypercholesterolemia .
swell
literature
- Charles Thom, Kenneth B. Raper: A Manual of the Aspergilli . Williams & Wilkins, Baltimore 1945 (English, archive.org [accessed January 16, 2010]).
- Kenneth B. Raper, Dorothy I. Fennel: The Genus Aspergillus . Williams & Wilkins, Baltimore 1965 (English).
- Robert A. Samson, John I. Pitt (Eds.): Advances in Penicillium and Aspergillus Systematics . Plenum Press, New York 1985, ISBN 0-306-42222-0 (English).
- Keith A. Powell, Annabel Renwick, John F. Peberdy (Eds.): The Genus Aspergillus: From Taxonomy and Genetics to Industrial Application . Plenum Press, New York 1994, ISBN 0-306-44701-0 (English).
- Gustavo H. Goldman, Stephen A. Osmani (Eds.): The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods . CRC Press, Boca Raton 2007, ISBN 978-0-8493-9080-7 (English).
- Masayuki Machida, Katsuya Gomi (Ed.): Aspergillus: Molecular Biology and Genomics . Caister Academic Press, Norwich 2010, ISBN 978-1-904455-53-0 (English).
- János Varga, Robert A. Samson (Eds.): Aspergillus in the Genomic Era . Wageningen Academic Publishers, Wageningen 2008, ISBN 978-90-8686-065-4 (English).
Web links
- The Aspergillus / Aspergillosis website. The Fungal Research Trust, accessed March 18, 2010 .
Individual evidence
- ↑ APJ Trinci, NR Morris: Morphology and Growth of a Temperature-sensitive mutant of Aspergillus nidulans Which Forms Aseptate mycelia at non-permissive Temperatures . In: Journal of General Microbiology . tape 114 , 1979, pp. 53-59 , doi : 10.1099 / 00221287-114-1-53 (English).
- ↑ APJ Trinci: A Kinetic Study of the Growth of Aspergillus nidulans and Other Fungi . In: Journal of General Microbiology . tape 57 , 1969, p. 11-24 , doi : 10.1099 / 00221287-57-1-11 , PMID 5822157 (English).
- ↑ Maren A. Klich: Biogeography of Aspergillus species in soil and litter . In: Mycologia . tape 94 , no. 1 , 2002, p. 21–27 (English, mycologia.org [PDF]).
- ↑ Eldor Alvin Paul (Ed.): Soil microbiology, ecology, and biochemistry . 3. Edition. Academic Press, Riverport 2007, ISBN 978-0-12-546807-7 , pp. 154 (English).
- ↑ Serena Ruisi, Donatella Barreca, Laura Selbmann, Laura Zucconi, Silvano Onofri: Fungi in Antarctica . In: Reviews in Environmental Science and Biotechnology . tape 6 , December 2007, pp. 127-141 , doi : 10.1007 / s11157-006-9107-y (English).
- ^ A b Alisa P. Alker, Garriet W. Smith, Kiho Kim: Characterization of Aspergillus sydowii (Thom et Church), a fungal pathogen of Caribbean sea fan corals . In: Hydrobiologia . tape 460 , no. 1–3 , September 2001, pp. 105-111 , doi : 10.1023 / A% 3A1013145524136 (English).
- ↑ John Mullins: Aspergillus and Aerobiology . In: Keith A. Owell, Annabel Renwick, John F. Pederby (Eds.): The Genus Aspergillus . Plenum Press, New York 1994, ISBN 0-306-44701-0 , pp. 351-359 (English).
- ↑ Bernard E. Proctor, Basil W. Parker: Microbiology of the Upper Air. III. An Improved Apparatus and Technique for Upper Air Investigations . In: Journal of Bacteriology . tape 36 , no. 2 , August 1938, p. 175-185 , PMID 16560151 (English).
- ^ A b Celine M. O'Gorman, Hubert T. Fuller, Paul S. Dyer: Discovery of a sexual cycle in the opportunistic fungal pathogen Aspergillus fumigatus . In: Nature . tape 457 , no. 29 , March 2009, p. 471-474 , doi : 10.1038 / nature07528 , PMID 19043401 (English).
- ^ A. Hocking: Responses of xerophilic fungi to changes in water activity . In: DH Jennings (Ed.): Stress Tolerance of Fungi . Marcel Dekker, New York 1993, ISBN 0-8247-9061-8 , pp. 233-256 (English).
- ↑ AP Torzilli, JD Isbister: Comparison of coal solubilization by bacteria and fungi . In: biodegradation . tape 5 , no. 1 , March 1994, p. 55-62 , doi : 10.1007 / BF00695214 (English).
- ^ Tristan Brandhorst, Patrick F. Dowd, William R. Kenealy: The effect of fungal ribosome inactivating proteins upon feeding choice in C. freemani, and indications of a mutualistic relationship with A. restrictus. Environmental Mycology . In: Mycopathologia . tape 152 , no. 3 , December 2001, p. 155-158 , doi : 10.1023 / A: 1013131930192 , PMID 11811644 (English).
- ↑ SR Loschiavo, RN Sinha: Feeding, Oviposition, and Aggregation by the Rusty Grain Beetle, Cryptolestes ferrugineus (Coleoptera: Cucujidae) on Seed-Borne Fungi . In: Annals of the Entomological Society of America . tape 59 , no. 3 , May 1966, pp. 578-585 , doi : 10.1093 / aesa / 59.3.578 (English).
- ^ DT Wicklow, PF Dowd, JB Gloer: Antiinsectan effects of Aspergillus metabolites . In: Keith A. Powell, Annabel Renwick, John F. Peberdy (Eds.): The Genus Aspergillus: From Taxonomy and Genetics to Industrial Application . Plenum Press, New York 1994, ISBN 0-306-44701-0 , pp. 93–114 (English, limited preview in Google Book Search). Keith A. Powell, Annabel Renwick, John F. Peberdy, Federation of European Microbiological Societies (Eds.): The Genus Aspergillus: from taxonomy and genetics to industrial application . Plenum Press, New York 1994.
- ↑ Anne D. van Diepeningen, János Varga, Rolf E. Hoekstra, Alfons JM debits: Mycoviruses in the Aspergilli . In: János Varga, Robert A. Samson (Eds.): Aspergillus in the Genomic Era . Wageningen Academic Publishers, Wageningen 2008, ISBN 978-90-8686-065-4 (English).
- ^ Geoffrey Clough Ainsworth: Introduction to the history of mycology . Cambridge University Press, Cambridge 1976, ISBN 0-521-21013-5 , pp. 175 (English, limited preview in Google Book Search).
- ↑ Axel Arthur Brakhage: Systemic fungal infections caused by Aspergillus species: epidemiology, infection process and virulence determinants . In: Current Drug Targets . tape 6 , no. 8 , 2005, p. 875-886 , PMID 16375671 (English).
- ^ William Kaplan, Paul Arnstein, Libero Ajello, Francis Chandler, John Watts, Martin Hicklin: Fatal aspergillosis in imported parrots . In: Mycopathologia . tape 56 , no. 1 , January 1975, p. 25-29 , doi : 10.1007 / BF00493579 (English).
- ↑ Robert Georg Markus: Studies on the therapy of air sac mycosis in horses - ligation of the internal carotid artery using transendoscopic clip application . University of Veterinary Medicine Hannover, Hannover 2002 ( elib.tiho-hannover.de [PDF] dissertation).
- ↑ Raper & Fennel, p. 95 f.
- ↑ I. Vennewald, J. Schönlebe, E. Klemm: Mycological and histological investigations in humans with middle ear infections . In: Mycoses . tape 46 , no. 1–2 , February 2003, pp. 12-18 , doi : 10.1046 / j.1439-0507.2003.00835.x (English).
- ^ Marianne Abele-Horn: Antimicrobial Therapy. Decision support for the treatment and prophylaxis of infectious diseases. With the collaboration of Werner Heinz, Hartwig Klinker, Johann Schurz and August Stich, 2nd, revised and expanded edition. Peter Wiehl, Marburg 2009, ISBN 978-3-927219-14-4 , pp. 197 and 282.
- ↑ Recommendation of the Commission of August 11, 2003 on the prevention and reduction of patulin contamination of apple juice and apple juice ingredients in other beverages , EU law
- ^ Raper & Fennel, p. 105.
- ↑ Raper & Fennel, p. 105 f.
- ↑ Raper & Fennel, pp. 106-109.
- ↑ Gottfried Kirchner: The curse of the Pharaoh - The secret knowledge of the ancient Egyptians . In: TERRA X puzzles of ancient world cultures . Bertelsmann, Frankfurt 1986, p. 24, 36 .
- ↑ János Varga, Akos Juhasz, Ferenc Kevei, Zofia Kozakiewicz: Molecular diversity of agriculturally important Aspergillus species . In: European Journal of Plant Pathology . tape 110 , no. 5-6 , June 2004, pp. 627-640 , doi : 10.1023 / B: EJPP.0000032402.36050.df (English).
- ↑ G. Perrone, A. Susca, G. Cozzi, K. Ehrlich, J. Varga, JC Frisvad, M. Meijer, P. Noonim, W. Mahakarnchanakul, RA Samson: Biodiversity of Aspergillus species in some important agricultural products . In: Studies in Mycology . tape 59 , no. 1 , 2007, p. 53-66 , doi : 10.3114 / sim.2007.59.07 , PMID 18490950 (English).
- ↑ C. Elizabeth Oakley, Berl R. Oakley: Identification of γ-tubulin, a new member of the tubulin superfamily encoded by mipA gene of Aspergillus nidulans . In: Nature . tape 338 , no. 6217 , 1989, pp. 662-664 , doi : 10.1038 / 338662a0 , PMID 2649796 (English).
- ↑ Dr. Kerstin Mauth: New genome decoding will greatly advance research. DFG Research Center for Molecular Physiology of the Brain, December 21, 2005, accessed on October 29, 2012 .
- ^ Joan W. Bennett: An Overview of the Genus Aspergillus . In: Masayuki Machida, Katsuya Gomi (ed.): Aspergillus: Molecular Biology and Genomics . Caister Academic Press, Norwich 2010, ISBN 978-1-904455-53-0 , pp. 1-18 (English).
- ↑ Andrew Breakspear, Michelle Momany: Microarrays in Aspergillus Species . In: Gustavo H. Goldman, Stephen A. Osmani (Eds.): The Aspergilli: Genomics, Medical Aspects, Biotechnology, and Research Methods . CRC Press, Boca Raton 2007, ISBN 978-0-8493-9080-7 , pp. 475-481 (English).
- ↑ Michael Köneke: Mold in the house: Recognize - avoid - fight. Risks, influencing factors, measurement methods, legal disputes, measures to control mold . Fraunhofer Irb, Sttagart 2008, ISBN 978-3-8167-7295-8 , p. 12 ( limited preview in Google Book search).
- ^ David Malloch: The Trichocomaceae: Relationships with other Ascomycetes . In: Robert A. Samson, John I. Pitt (Eds.): Advances in Penicillium and Aspergillus Systematics . Plenum Press, New York 1985, ISBN 0-306-42222-0 , pp. 365-382 (English).
- ↑ Mary L. Berbee, Atsuko Yoshimura, Junta Sugiyama, John W. Taylor: Is Penicillium monophyletic? An Evaluation of Phylogeny in the Family Trichocomaceae from 18S, 5.8S and ITS Ribosomal DNA Sequence Data . In: Mycologia . tape 87 , no. 2 (March – April), 1995, pp. 210-222 , JSTOR : 3760907 (English).
- ↑ Junta Sugiyama: Relatedness, phylogeny, and evolution of the fungi . In: Mycoscience . tape 39 , no. 4 , December 1998, pp. 487-511 , doi : 10.1007 / BF02460912 (English).
- ↑ M. Tamura, K. Kawahara, J. Sugiyama: Molecular phylogeny of Aspergillus and associated teleomorphs in the Trichocomaceae (Eurotiales) . In: Robert A. Samson, John I. Pitt (Eds.): Integration of Modern Taxonomic Methods For Penicillium and Aspergillus Classification . CRC Press, Boca Raton 2000, ISBN 90-5823-159-3 , pp. 357-373 (English).
- ↑ JI Pitt, RA Samson: Nomenclatural considerations in naming species of Aspergillus and its teleomorphs . In: Studies in Mycology . tape 59 , 2007, p. 67-70 , doi : 10.3114 / sim.2007.59.08 , PMID 18490944 (English).
- ↑ CP Kurtzman, MJ Smiley, CJ Robnett, DT Wicklow: DNA Relatedness among Wild and Domesticated Species in the Aspergillus flavus Group . In: Mycologia . tape 78 , no. 6 (November – December), 1986, pp. 955-959 , JSTOR : 3807436 (English).
- ↑ a b Heinrich Dörfelt, Alexander R. Schmidt: A fossil Aspergillus from Baltic amber . In: Mycological Research . tape 109 , no. 8 , August 2005, p. 956-960 , doi : 10.1017 / S0953756205003497 , PMID 16175799 (English).
- ^ Index Fungorum. Retrieved January 16, 2010 .
- ^ The Aspergillus website. Retrieved January 16, 2010 .
- ↑ V. Robert, G. Stegehuis, J. Stalpers: The MycoBank engine and related databases. Retrieved January 16, 2010 .
- ^ Robert A. Samson: A compilation of the Aspergilli described since 1965 . In: Studies in Mycology . tape January 18 , 1979 ( cbs.knaw.nl [accessed March 9, 2010]).
- ^ JC Neill: The Mold Fungi of New Zealand. II.The genus Aspergillus . In: Transactions and Proceedings of the Royal Society of New Zealand 1868-1961 . tape 69 , 1940, pp. 237–264 (English, rsnz.natlib.govt.nz [accessed March 11, 2010]).
- ↑ RA Samson1, S. Hong, SW Peterson, JC Frisvad, J. Varga: Polyphasic taxonomy of Aspergillus section Fumigati and its Teleomorph Neosartorya . In: Studies in Mycology . tape 59 , no. 1 , 2007, p. 147–203 , doi : 10.3114 / sim.2007.59.14 , PMID 18490953 (English).
- ↑ JL Varshney, AK Sarbhoy: A new species of Aspergillus fumigatus group and comments upon its synonymy . In: Mycopathologia . tape 73 , no. January 2 , 1981, pp. 89-92 , doi : 10.1007 / BF00562596 , PMID 7012634 (English).
- ^ Robert A. Samson, Seung-Beom Hong, Jens C. Frisvad: Old and new concepts of species differentiation in Aspergillus . In: Medical Mycology . tape 44 , s1, 2006, p. 133-148 , doi : 10.1080 / 13693780600913224 (English).
- Jump up ↑ Yoshikazu Horie, Paride Abliz, Kazutaka Fukushima, Kaoru Okada, GM Campos Takaki: Two new species of Neosartorya from Amazonian soil, Brazil . In: Mycoscience . tape 44 , no. 5 , October 2003, p. 397-402 , doi : 10.1007 / s10267-003-0132-1 (English).
- ↑ MA Klich: Morphological Studies of Aspergillus Section Versicolores and Related Species . In: Mycologia . tape 85 , no. 1 (January – February), 1993, pp. 100-107 , JSTOR : 3760484 (English).
- ↑ RA Samson, J. Mouchacca: Additional notes on species of Aspergillus, eurotium and Emericella from Egyptian desert soil . In: Antonie van Leeuwenhoek . tape 41 , no. 3 , 1975, p. 343-351 , doi : 10.1007 / BF02565069 , PMID 1082299 (English).
- ↑ MA Ismail, AA Zohri: Confirmation of the relationships of Aspergillus nidulans egyptiacus and Emericella using progesterone transformation . In: Letters in Applied Microbiology . tape 18 , no. 3 , June 2008, p. 130-131 , doi : 10.1111 / j.1472-765X.1994.tb00825.x (English).
- ↑ Janos Varga, Jos Houbraken, Henrich AL Van Der Lee, Paul E. Verweij, Robert A. Samson: Aspergillus calidoustus sp. nov., Causative Agent of Human Infections Previously Assigned to Aspergillus ustus . In: Eukaryotic Cell . tape 7 , no. 4 , April 2008, p. 630-638 , doi : 10.1128 / EC.00425-07 , PMID 18281596 (English).
- ↑ JC Frisvad, P. Skouboe, RA Samson: Taxonomic comparison of three different groups of aflatoxin producers and a new efficient producer of aflatoxin B1, sterigmatocystin and 3-O-methylsterigmatocystin, Aspergillus rambellii sp. nov. In: Systematic and Applied Microbiology . tape 28 , no. 5 , July 2005, p. 442-453 , PMID 16094871 (English).
- ↑ S. Arunmozhi Balajee, John W. Baddley, Stephen W. Peterson, David Nickle, Janos Varga, Angeline Boey, Cornelia Lass-Florl, Jens C. Frisvad, Robert A. Samson: Aspergillus alabamensis, a New Clinically Relevant Species in the Section Terrei . In: Eukaryotic Cell . tape 8 , no. 5 , May 2009, p. 713-722 , doi : 10.1128 / EC.00272-08 , PMID 19304950 (English).
- ^ Jens C. Frisvad, Mick Frank, Jos AMP Houbraken, Angelina FA Kuijpers, Robert A. Samson: New ochratoxin A producing species of Aspergillus section Circumdati . In: Studies in Mycology . tape 50 , no. 1 , 2004, p. 23-43 (English).
- ↑ Martha Christensen: The Aspergillus ochraceus group: two new species from western soils and a synoptic key . In: Mycologia . tape 74 , no. 2 , 1982, p. 210-225 , JSTOR : 3792887 (English).
- ↑ Dong Mei Li, Yoshikazu Horie, Yuxin Wang, Rouyu Li: Three new Aspergillus species isolated from clinical sources as a causal agent of human aspergillosis . In: Mycoscience . tape 39 , no. 3 , October 1998, p. 1340-3540 , doi : 10.1007 / BF02464012 (English).
- ↑ Yoko Ito, SW Peterson, T. Goto: Properties of Aspergillus tamarii, A. caelatus and related species from acidic tea field soils in Japan . In: Mycopathologia . tape 144 , no. 3 , December 1998, pp. 169-175 , doi : 10.1023 / A: 1007021527106 , PMID 10531683 (English).
- ↑ Bruce W. Horn, Jorge H. Ramirez-Prado, Ignazio Carbone: Sexual reproduction and recombination in the aflatoxin-producing fungus Aspergillus parasiticus . In: Fungal Genetics and Biology . tape 46 , no. 2 , February 2009, p. 169–175 , doi : 10.1016 / j.fgb.2008.11.004 , PMID 19038353 (English).
- ^ Robert A. Samson, Jos AMP Houbraken, Angelina FA Kuijpers, J. Mick Frank, Jens C. Frisvad: New ochratoxin A or sclerotium producing species in Aspergillus section Nigri . In: Studies in Mycology . tape 50 , no. 1 , 2004, p. 45-61 (English).
- ↑ T. Inui: Investigations into the lower organisms which participate in the production of the alcoholic drink "Awamori" . In: Journal of the College of Science . 1901, p. 469-484 ( repository.dl.itc.u-tokyo.ac.jp [PDF]). repository.dl.itc.u-tokyo.ac.jp ( Memento of the original from May 25, 2015 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ↑ Paramee Noonim, Warapa Mahakarnchanakul, Janos Varga, Jens C. Frisvad, Robert A. Samson: Two novel species of Aspergillus section Nigri from Thai coffee beans . In: International Journal of Systematic and Evolutionary Microbiology . tape 58 , 2008, p. 1727–1734 , doi : 10.1099 / ijs.0.65694-0 , PMID 18599725 (English).
- ↑ Donatella Mares, Elisa Andreotti, Maria Elena Maldonado, Paola Pedrini, Chiara Colalongo, Carlo Romagnoli: Three New Species of Aspergillus from Amazonian Forest Soil (Ecuador) . In: Current Microbiology . tape 57 , July 2008, p. 222-229 , doi : 10.1007 / s00284-008-9178-9 , PMID 18594910 (English).
- ↑ Oriana Magii, Anna Maria Persiani: Aspergillus implicatus, a new species isolated from Ivory Coast forestsoil . In: Mycological Research . tape 98 , no. 8 , 1994, pp. 869-873 (English).
- ↑ Takashi Yaguchi, Ayako Someya and Shun-ichi Udagawa: Aspergillus taichungensis, a new species from Taiwan . In: Mycoscience . tape 36 , no. 4 , December 1995, pp. 1618-2545 , doi : 10.1007 / BF02268626 (English).
- ^ H. Benninga: A History of Lactic Acid Making: A Chapter in the History of Biotechnology . Springer, New York 1990, ISBN 0-7923-0625-2 , pp. 140 ff . ( limited preview in Google Book search).
- ↑ a b Bernard Dixon: Aspergillus niger - the end of an Italian monopoly . In: The Mushroom That Made John F. Kennedy President . Spectrum, Heidelberg 1995, ISBN 3-86025-289-5 , p. 63-66 .
- ^ David R. Dodds, Richard A. Gross: Chemicals from Biomass . In: Science . tape 318 , no. 5854 , November 2007, p. 1250–1251 , doi : 10.1126 / science.1146356 , PMID 18033870 (English).
- ↑ Enzyme List. (PDF) (No longer available online.) Association of Manufacturers and Formulators of Enzyme Products (AMFEP), 2009, archived from the original on May 25, 2015 ; Retrieved March 18, 2010 . Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice.
- ^ Piet WM van Dijck: The importance of Aspergilli and regulatory aspects of Aspergillus nomenclature in biotechnology . In: János Varga, Robert A. Samson (Eds.): Aspergillus in the Genomic Era . Wageningen Academic Publishers, Wageningen 2008, ISBN 978-90-8686-065-4 , p. 249-256 (English).