Ribosomal DNA
As ribosomal DNA (rDNA) are those portions of the deoxyribonucleic acid (DNA) indicated that the genes for ribosomal RNA containing (rRNA). rRNA is an important part of the ribosomes , in which protein synthesis ( translation ) takes place. The rRNA is therefore a structural ribonucleic acid (RNA), the information of which is not transcribed into proteins.
In eukaryotic cells , rDNA is not only found in the nucleus, but also in the mitochondria and, in plants, also in the plastids . The rRNA they read is only used in the ribosomes of these organelles , not in the cytoplasm. Mitochondrial and plastid rDNA is similar in sequence to the rDNA in prokaryotes , which supports the endosymbiotic theory .
Eukaryotic cells require large amounts of rRNA. Therefore rDNA occurs in many copies. Tandem copies, a type of repetitive DNA, are typical of rDNA . The proportion of rDNA in the genome is different for different organisms. In humans it is about 0.4%, on 10 of the 46 chromosomes , in Drosophila melanogaster about 17%.
Not all chromosomal segments containing rDNA are always read and used for rRNA synthesis in all cells. The active rDNA sections are concentrated in one or a few dense areas in the cell nucleus , the nucleoli (also: nucleoli; singular: nucleolus) or nuclear bodies. The sections containing rDNA are therefore also referred to as " nucleolus organizing regions " (NORs). Prokaryotes, mitochondria and plastids do not form a nucleolus.
rDNA in prokaryotes
Although archaea and bacteria form their own kingdoms , their rRNA and rDNA are organized in a similar way. Both types of prokaryotes typically have only three different rRNAs: 16S, 23S, and 5S rRNA. Their genes are often clustered together and transcribed together , so they form an operon .
bacteria
The number of rDNA operons in bacteria can vary, e.g. B. two for Mollicutes , seven for Escherichia coli or ten for Bacillus subtilis . The operons are usually designated with continuous letters (for E. coli : rrnA to rrnG). Often, for example in E. coli , the rDNA cluster also contains genes for tRNAs . Which and whether tRNA genes are included is very variable.
The primary transcript of the prokaryotic rDNA is called 30S pre-rRNA. The 16S and 23S rRNAs are cut out by ribonuclease III. The “fine cut” and the processing of the 5S rRNA and the tRNAs are carried out by further RNA-cutting enzymes. So that the functional rRNA areas are not also cut, they are methylated immediately after transcription and thus protected.
Due to the great diversity of bacteria, there are also special developments. In mycoplasma , for example, the 5S rDNA is separate from the 16S and 23S rDNA. The Planctomycete Planctomyces limnophilus has two sets of ribosomal genes, the 16S rDNA is separate from the closely spaced clusters of 23S and 5S rDNA.
Archaea
In some archaea, the 5S rDNA is a little further away from the other two rDNAs. Sometimes (e.g. with Thermococcus celer ) there is also another 5S rDNA a few 1000 base pairs away.
rDNA in eukaryotic organelles
The rDNAs of mitochondria and plastids are principally similar to prokaryotes, but have many special developments.
Plastids
The structure of the ribosomes, the basic sequence of the rRNA genes (16S-23S-5S) and their sedimentation coefficient (70S) are similar to prokaryotes. There are also tRNA genes in the transcription unit in the chloroplast rDNA. The rDNA clusters can have considerable differences in terms of length, the tRNA genes and also the introns within the rRNA genes. It is sometimes speculated that the chloroplast genomes of the large plant groups could be of different origins.
In higher plants such as tobacco ( Nicotiana tabacum ) and rice ( Oryza sativa ), a 4.5S rDNA was described between the 5S and the 23S rDNA, deviating from the basic structure. This is homologous to the 3 'end of the 23S rDNA. The 4,5S rRNA, along with the 23S and 5S rRNA, is part of the large subunit of ribosomes. The green alga Chlamydomonas reinhardtii has a 7S and a 3S rDNA between the 16S and 23S rDNA. This combination is unique in this alga.
The rDNA is often located in the so-called "inverted repeats", two long, opposing repetitions, e.g. B. in the original Glaucocystaceae Cyanophora paradoxa , the Olisthodiscus luteus ( Raphidophyceae ) and most green plants. In many butterfly plants and in pines the inverted repeat has been lost secondarily. There are no inverted repeats in the red alga Porphyra purpurea and Euglena gracilis . In Euglena there are three repeats and an additional 16S rDNA in direct repeat units. In some Chromalveolata , e.g. B. in dinoflagellates and brown algae , such as Pylaiella littoralis , there are several plastid chromosome rings. In P. littoralis the rDNA is on a ring as in green plants in inverted repeats . In the small chromosome ring there is a 16S pseudogene and a distant 23S rDNA gene.
Mitochondria
The arrangement of the rDNA in mitochondria is very heterogeneous. It is often heavily modified from the prokaryotic form, so that at times the hypothesis was put forward that the endosymbiosis of mitochondria occurred several times during evolution.
Usually, as in humans, there are only two rRNA genes left, one each for the rRNA of the large and the small subunit of the ribosomes. Both genes can sometimes be distant from each other. The rRNAs formed are significantly smaller than those of the prokaryotes: in animals 12S in the small ribosome subunit and 16S in the large, in fungi 15S in the small and 21S in the large subunit.
An independent version similar to the 5S rDNA has only been described in a few green algae (e.g. Prototheca wickerhamii ), in land plants and in the single cell Reclinomonas americana . Reclinomonas is considered to be the most pristine eukaryote that possesses mitochondria. In red algae, on the other hand, which are evolutionarily closer to green plants than they are, the 5S rDNA can no longer be detected.
The rDNA is only present in the form of an operon in land plants. In green algae such as Chlamydomonas reinhardtii , the rDNA genes are fragmented and also interrupted by other genes. Even with Reclinomonas , the organization as an operon is no longer clear.
rDNA in the nucleus of eukaryotes
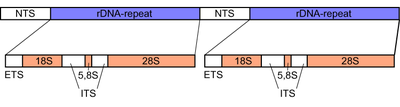
The cytoplasmic ribosomes of the eukaryotes contain four different rRNAs. These can make up up to 90% of the total RNA of a cell. For the production of such large quantities, many genes are required for each of the four rRNAs. The genes of three (those of the 18S-, 5.8S- and 28S-rRNA) are located directly one behind the other and are transcribed together by the RNA polymerase I. Such transcription units lie in large numbers as tandem-like repeating units (English: Repeats ), they form the actual rDNA (see figure). In the microscopic image of the metaphase , the repeating units can be seen as secondary constrictions, since the rDNA is particularly tightly packed.
The genes of the 5S rRNA form their own gene family, which strictly speaking does not belong to the rDNA. They are transcribed by RNA polymerase III. In the case of some yeasts , such as Hansenula polymorpha or Saccharomyces cerevisiae , they are located directly behind the 18S, 5.8S, 28S repeats, but on the opposite strand, i.e. in the opposite reading direction. In most of the other yeasts and the rest of the eukaryotes, the 5S rRNA genes are present apart from the 18S, 5.8S, and 28S repeats. They also often form tandem repeats.
Exactly the same number of all rRNAs is required for the synthesis of the ribosomes. Due to the spatial separation of the 5S rRNA genes, the eukaryotes (in contrast to the prokaryotes) do not automatically produce the same amount, so that special regulation is required (see below ).
The rDNA is usually part of the chromosomes . It is extrachromosomal only in some protists , such as the slime mold genus Physarum .
18S, 5.8S, 28S rDNA clusters

The 18S, 5.8S and 28S rRNA genes of a transcription unit are separated at the DNA level by two so-called internal transcribed spacers (ITS) and listed together by an external transcribed spacer (ETS) (see diagram). Sometimes there is also an ETS at the repeat end. Successive transcription units are separated by non-transcribed spacers (NTS).
The non-transcribed spacers in front of individual repeat blocks consist of a multiplication of promoters (a total of 2300 to 5300 bp ), so that they have a high binding force for the RNA polymerases. The high promoter effectiveness means that the rDNA genes are transcribed multiple times at the same time, which is reflected in the so-called " Miller spread " through "fir tree structures" (see picture).
First of all, the external transcribed spacer is separated from the 45S pre-rRNA, resulting in the 41S rRNA precursor. This is split into a 20S pre-rRNA and a 32S precursor in the first ITS. In the third step, the small ITS part is separated from the 20S precursor and the mature 18S rRNA is created. At the same time, the ITS are cut out from the 32S precursor and the 5.8S rRNA is bound to the complementary central part of the 28S rRNA. These reactions are catalyzed by so-called small nucleolar ribonucleoprotein particles ( snoRNP ).
In Drosophila , but also in many other organisms, the 28S rDNA can contain a so-called intervening sequence (IVS). This is assigned to the “Group I introns ”, which splice autocatalytically . An external guanosine monophosphate is linked to the 3'-OH of the start of the intron and the 3 'end of the first exon that has now been released to the 5' end of the second exon. This releases and breaks down the intron and links the exons. However, it has not yet been clarified when this splicing takes place during the rRNA procession.
The arrangement, distribution and number of transcription units can vary greatly depending on the species . Drosophila males have around 150 copies, the females 250. 22,000 copies have been described for the field bean ( Vicia faba ).
In humans, the approximately 43 kb long 18S-5.8S-28S transcription units are arranged in tandem to form 30 or more clusters. The total of ten clusters are located on the acrocentric chromosome pairs 13, 14, 15, 21 and 22. The total number of transcription units is estimated at 500 copies. Each transcription unit has its own complex regulated promoter . There are so-called spacer promoters in NTS areas at regular intervals between the repeat units per cluster. These transcribe a noncoding RNA, which regulates the transcription of the individual transcription units via a protein complex called NoRC.
5S rRNA genes
5S rRNA genes are also present in tandem repeats. In humans there is a block on the long arm of chromosome 1 near the telomere , in Drosophila melanogaster there is a block on chromosome 3. The clawed frog ( Xenopus laevis ) has about 24,000 copies near the ends of most of the chromosomes. The DNA sections between 5S rRNA genes are also called non-transcribed spacers . They can vary greatly both between individual gene copies and between closely related species.
In contrast to the other rRNA genes, but like the tRNAs , 5S rRNA genes are transcribed by RNA polymerase III. They have no introns and are not processed . The promoter lies within the coding sequence and is called the internal control region . It consists of a box A, an intermediate element and a box C (from 5 'to 3').
The 5S rRNA genes of the clawed frog differ within an individual in that some are only active in egg cells , while others are only active in somatic cells.
Regulation of the rRNA content
Eukaryotic cells must ensure that 5S-RNA and the other RNAs are produced in the same quantities. A different processivity of the RNA polymerases I and III is compensated, among other things, by different copy numbers of the rRNA genes. In addition, the different structure of the respective NTS regions can also lead to differential regulation. In certain situations only part of the cellular rDNA is transcribed.
In some situations, however, the total content of the rDNA genes must be changed. The polytene chromosomes of Drosophila , the rDNA is unterrepliziert for example. More often, however, it is necessary to produce more ribosomes, for example in egg cells. This is made possible by intracellular replication ( amplification ) of the genes. In this case, the rDNA genes are excised from the chromosome, and circularized by rolling circle - replication amplified. These strongly stainable DNA rings, first described in 1901, are called Giardina bodies after their discoverer . They have been observed in the yellow beetle , the house cricket , the clawed frog and the hamster . The molecular mechanism is still unclear.
Ribosomal DNA and Evolution
Ribosomes are complex and vital components of every cell, so the conservation is quite high. In contrast to prokaryotes, eukaryotes have several hundred copies, so that an independent evolution could be expected. However, the clusters are homogenized by inequitable crossing over and thus inherited like a single gene. In the case of hybrids that have both genomes of the parent parents (allotetraploid), only the rDNA of one parent is transcribed, which is known as “ nucleolar dominance ”.
Evolution of rDNA
The repeat structure of the rDNA is particularly striking. It is found in bacteria, archaea, in the eukaryotic cytoplasm and some (plant) mitochondrial DNAs. So is quite catchy, that the 16S rDNA with the 18S rDNA and 23S rDNA with the 28S rDNA homologisierbar are. The 5.8S rDNA is homologous to the end of the 23S rDNA of prokaryotes. The 5S rDNA of prokaryotes is homologous to the 5S rDNA of eukaryotes.
As the rDNA in mitochondria in particular shows, various changes in the arrangement can occur without the functionality of the ribosomes having to suffer. Since the exact interactions of the rRNAs are not yet sufficiently understood, many questions remain open.
Evolution research with rDNA
The importance of rDNA is also very interesting for evolutionary researchers, because defective gene copies are predominantly harmful, and that for all protein syntheses in the cell, and are selected out . In addition, ribosomes are present in all living things, and it is even possible to build bridges between prokaryotes and eukaryotes. So z. B. the 16S rDNA is used to systematize large groups of bacteria . But even in the large systematics of the eukaryotes, the sequences of the mature rRNA of the ribosome subunits are definitely a popular marker. The plastid and mitochondrial rDNA is also widely used.
When comparing the ribosomal DNAs between prokaryotes and eukaryotes, the sequences are only slightly identical. The folds of the rRNAs have an enormous morphological similarity despite the different base composition.
The ITS sequences that are not directly influenced by selection play an important role in phylogeny at the level of genus and species . Usually, the 5.8S rDNA is also sequenced at the same time, as it is hardly significant in terms of size. In order to obtain greater statistical certainty, the ITS sequences are supplemented by at least one additional marker. The use of ITS sequences has meanwhile decreased, on the one hand because other markers of the cell nucleus are becoming more available and on the other hand because the use of ITS poses greater problems for the molecular phylogeneticists . The individual bases are not conserved, but structural elements such as folds, so that the mutations are not free. The adaptation of different ITS copies in hybrids can also take place in just a few generations, so that ITS can behave phylogenetically similar to chloroplasts. Nevertheless, the high number of copies and thus the better availability in the laboratory is a good argument to continue using ITS.
history
Ribosomes from eukaryotes consist of four different rRNAs, each of which occurs in equal numbers. Cyrus Levinthal et al. found out in 1962 that rRNAs and tRNAs are transcribed by DNA and cannot replicate on their own. In 1965, Sol Spiegelman and Ferrucio Ritossa showed by hybridizing rRNA with Drosophila cell nuclei with different numbers of nucleolus organizer regions that these regions contain the coding rDNA.
Carl Woese published his tree of life in 1977 , a family tree that was able to bring all large groups together for the first time. This was based on sequences of the 16S rDNA from mitochondria and presented the archaea as a separate realm .
In 1982 Thomas R. Cech discovered the intervening sequence in the 28S rRNA of the ciliate Tetrahymena thermophila , an autocatalytically splicing intron . For this groundbreaking discovery that RNA can also take on enzymatic functions and splice itself, he and Sidney Altman received the Nobel Prize in Chemistry in 1989 .
Individual evidence
- ↑ J. Brosius, TJ Dull, DD Sleeter, HF Noller: Gene Organization and Primary Structure of a Ribosomal RNA Operon from "Escherichia coli". In: J. Mol. Biol. 148/1981, pp. 107-127
- ↑ C. Taschke, M.-Q. Klinkert, J. Wolters, R. Herrmann: Organization of the ribosomal RNA genes in "Mycoplasma hyopneumoniae": The 5S rRNA gene is separated from the 16S and 23S rRNA genes. In: Mol Gen Genet. 205/1986, pp. 428-433
- ^ N. Ward-Rainey, FA Rainey, EMH Wellington, E. Stackebrandt: Physical Map of the Genome of "Planctomyces limnophilus", a Representative of the Phylogenetically Distinct Planctomycete Lineage. In: Journal of Bacteriology. April 1996, pp. 1908-1913
- ↑ H. Neumann, A. Gierl, J. Tu, J. tunic, D. Staiger, W. Zillig: Organization of the gene for ribosomal RNA in archaebacteria. In: Mol Gen Genet. 192/1983, pp. 66-72
- ↑ Z. Schwarz, H. Koessel: The primary structure of 16 S rDNA from "Zea mays" chloroplast is homologous to "E. coli “16-S rRNA. In: Nature . 283/1980, pp. 739-742
- ↑ K. Edwards, J. Bedbrook, T. Dyer, H. Kössel: 4.5S rRNA from “Zea mays” chloroplasts shows structural homology with the 3'-end of prokaryotic 23S rRNA. In: Biochem. Int. 2/1981, pp. 533-538
- ↑ M. Sugiura: The chloroplast genome. In: Plant Molecular Biology. 19/1992, pp. 149-168
- ^ JD Palmer: Comparative organization of chloroplast genomes. In: Ann. Rev. Genet. 19/1985, pp. 325-354
- ^ CJ Howe, R. Ellen, R. Nisbet, AC Barbrook: The remarkable chloroplast genome of dinoflagellates. In: Journal of Experimental Botany. 59 (5), March 2008, pp. 1035-1045, doi : 10.1093 / jxb / erm292
- ↑ C. Leblanc, O. Richard, B. Kloareg: Origin and evolution of mitochondria: what have we learned from red algae? In: Current Genetics. 31/1997, pp. 193-207
- ↑ T. Schmidt, T. Schwarzacher, JS Heslop-Harrison: Physical mapping of rRNA genes by fluorescent in-situ hybridization and structural analysis of 5S rRNA genes and intergenic spacer sequences in sugar beet (Beta vulgaris). in: Theoretical and Applied Genetics edition. 88, pp. 629-636 (1994)
- ^ S. Johansen, T. Johansen, F. Haugli: Extrachromosomal ribosomal DNA of "Didymium iridis": sequence analysis of the large subunit ribosomal RNA gene and sub-telomeric region. In: Curr Genet. 22/1992, pp. 305-312
- ↑ SO Rogers, AJ Bendich: Ribosomal RNA genes in plants: variability in copy number and in the intergenic spacer. In: Plant Molecular Biology. 9 (5) / 1987, pp. 509-520
- ↑ Liao Daiqing, in: Encyclopedia of the human genome, in 2003, Macmillan Publishers LTd, Nature Publishing Group. pdf ( Memento from April 30, 2006 in the Internet Archive )
- ↑ N. Arnheim, M. Krystal, R, Schmickel, G. Wilson, O. Ryder, E. Zimmer: “Molecular evidence for genetic exchanges among ribosomal genes on nonhomologous chromosomes in man and apes” In: Proc. Natl. Acad. Sci. USA . 77 (12) / 1980, pp. 7323-7327
- ^ DM Hillis, MT Dixon: Ribosomal DNA: Molecular Evolution and Phylogenetic Inference. In: The Quarterly Review of Biology. 66 (4) / 1991, pp. 411-453
- ↑ T. Honjo, RH Reeder: Preferential transcription of Xenopus laevis ribosomal RNA in interspecies hybrids between Xenopus laevis and Xenopus mulleri. In: J. Mol. Biol. 80/1973, pp. 217-228
- ↑ ZJ Chen, CS Pikaard: Transcriptional analysis of nucleolar dominance in polyploid plants: biased expression / silencing of progenitor rRNA genes is developmentally regulated in Brassica. In: Proc. Natl. Acad. Sci. UNITED STATES. 94/1997, pp. 3442-3447
- ↑ PM Rubtsov, MM Musakhanov, VM Zakhaiyev, AS Krayev, KG Skryabin, AA Bayev: The structure of the yeast ribosomal RNA genes. I. The complete nudeotide sequence of the 18S ribosomal RNA gene from "Saccharomyces cerevisiae". In: Nucleic Acids Research . 8/23/1980, pp. 5779-5794
- ↑ GJ Olsen, DJ Lane, SJ Giovannoni, NR Pace: Microbial ecology and evolution: a ribosomal RNA approach. In: Ann. Rev. Microbiol. 40/1986, pp. 337-365
- ↑ C. Levinthal, A. Heynan, A. Higa: Messenger mRNA turnover and protein synthesis in "B. subtilis “inhibited by actinomycin D. In: Proc. Nat. Acad. Sci. 48/1962, pp. 1631-1638
- ↑ F. Ritossa, S. Spiegelman: Localization of DNA complementary to ribosomal RNA in the nucleolus oreganizer region of "Drosophila melanogaster". In: Proc. Nat. Acad. Sci. 53/1965, pp. 737-745
- ^ CR Woese, GE Fox: Phylogenetic Structure of the Prokaryotic Domain: The Primary Kingdoms. In: Proc. Nat. Acad. Sci. 74 (11) / 1977, pp. 5088-5090
literature
Prokaryotes
- AM Nedelcu: Fragmented and scrambled mitochondrial ribosomal RNA coding regions among green algae: a model for their origin and evolution. In: Mol Biol Evol. 14 (5) / 1997, pp. 506-517; PMID 9159928 .
- Seyffert: Textbook of Genetics. Spectrum, 2nd ed., 2003.
Eukaryotes
- Graw: Genetics. ; 4th edition, 2006 (formerly Hennig)
- L. Klabunde: Coproduction of pharmaceutical proteins and auxiliary factors to optimize microbial expression systems with restriction to a single integrative vector system . 1996
- N. Neves, M. Delgado: Ribosomal DNA heterochromatin in plants. Cytogenet Genome Res 109: 104-111 (2005); PMID 15753565 .