coal
Coal (from Altgerm. Kula , Old High German kolo , Middle High German kul and kol , "coal, charcoal") is a black or brownish-black, solid sedimentary rock that is formed by the carbonization of plant biomass . It mainly consists of the chemical element carbon and complex carbon compounds .
Coal is available on every continent. Its main geological period was the Upper Carboniferous ( Pennsylvania ) and the corresponding coal is mostly available as hard coal . In addition, there are also younger coal, for example in the Jura and the Chalk of Western Canada. In relation to Central Europe, the " Tertiary " is also an important coal formation period. However, this coal is predominantly in the form of lignite .
Coal is mainly used as a carrier of fossil energy . When they are burned , heat is released that can be used for heating . Burning coal is one of the most widely used techniques for generating electrical energy around the world . It is also important as a starting material in the manufacture of coke and graphite , the extraction of liquid hydrocarbons and iron smelting . The calorific value of a hard coal unit serves as a benchmark for other fuels.
Both coalification and coal burning are essential parts of the global carbon cycle .
The amount of global coal reserves is the subject of ongoing research. Estimates from the year 2004 calculate with constant consumption several hundred years until their exhaustion, other estimates assume that the maximum coal production could be reached in 2025. Delivery rates of the individual types of coal can be found under coal / tables and graphics .
Emergence
The raw material for coal is mainly of vegetable origin. Typical coal formation (humus coal, see general information on classification: types of coal ) begins in extensive swamp forests on lowlands . The trees use photosynthesis to bind carbon dioxide from the air and convert it into the carbohydrate cellulose and other organic compounds . After individual trees die, they sink into the swamp and are thus withdrawn from the normal aerobic decomposition process - initially peat is formed .
The geological history of the region in question is decisive for whether the peat is turned into coal. The earth's crust usually experiences a tectonic subsidence in the area of lowlands ( subsidence , see also sedimentary basins ). If this subsidence continues over geological periods of time (i.e. many tens of millions of years), the peaty swamp sediments, often under different environmental conditions (including long-lasting marine cover), are continuously overlaid with sediments and at the same time sunk into the deeper upper crust of the earth. Both the ambient pressure and the ambient temperature increase as the sinking depth increases . This causes the so-called coalification of the peaty sediments. The pressure squeezes out the water, and the increase in temperature causes the chemical conversion of the organic compounds, in the course of which an enrichment of carbon takes place. Initially, lignite is produced . With increasing subsidence, the coalification intensifies. Lignite becomes hard coal and finally anthracite . That is why the quality of coal is often better the deeper it lies in the ground and the older it is.
The most important hard coal deposits today (not infrequently only in terms of economic history ) were formed in the Upper Carboniferous around 323 to 299 million years ago. In the early Upper Carboniferous, the most important starting material for the peat in question consisted of 60 to 80% tree-like club moss plants . Their trunks were hardly made of wood , but had a very thick periderm (bark). In the middle upper carbon of the Appalachian Mountains , the heavily woody Cordaites dominated . In the late Upper Carboniferous Euramerica , weakly woody tree ferns from the Marattiales order again predominated. The economically important lignite deposits in Central Europe (Lower Rhine, Central Germany, Niederlausitz, Egergraben) were created in the " Tertiary " 66 to 2.6 million years ago and are therefore much younger.
Depending on the paleogeographical position of the educational area, a distinction is made between palustric (or limnic) and parali coal formations. Palustric / limnic is understood to mean coal formation in wetlands near inland waters. Parallel means that the coal deposit goes back to the formation of bog in a coastal plain. Between the individual coal seams, marine sediments are then repeatedly switched in, which can be traced back to brief transgressive phases. If coal deposits have their origin in Palustrian formation spaces within basins in mountainous regions, one can specifically speak of intramontaneous coal formation.
New studies suggest a close connection between the formation of the enormously rich carbonaceous coal deposits and the evolution of white rot , that is, of types of fungus that were able to break down lignin , a main component of wood. Molecular genetic relationship analyzes in connection with the method of the molecular clock showed that the white rot probably only arose at the end of the Carboniferous or in the early Permian .
Extraction
Coal can be extracted both above ground in open-cast mining and underground . Around 40% of the world's coal is extracted from open-cast mining, the rest from underground mining.
Stocks
The lignite reserves in Germany amounted to around 76.8 billion tons in February 2014, of which 40.3 billion tons could be economically extracted with today's technology. With constant production (2013: 183 million t), the reserves would be sufficient for 220 years.
Around 24 billion tons of German hard coal reserves are considered recoverable. Based on the production quota in 2004 (25.7 million tons), this would result in a theoretical range of over 900 years. Due to the geological conditions, however, only a part of these recoverable reserves can be mined in an internationally competitive manner according to the state of the art. Representatives of the German coal industry therefore put the range of German hard coal at around 400 years, assuming that the production volume at that time was maintained.
The German Energy Watch Group , an independent group of analysts led by scientists from the Ludwig-Bölkow -Stiftung (Munich), came to a different conclusion in spring 2007 with regard to global coal reserves and especially with regard to the reserve situation in Germany:
“Many statistics are out of date. […] Presumably, significantly less coal is available than is widely assumed. […] Much of the information has not been updated for years. Where this was done, the reserves were usually revised downwards, sometimes very drastically. ' The Federal Institute for Geosciences, for example, had stated the German hard coal reserves at 23 to 24 billion tons for decades. In 2004, they were downgraded to 183 million tons, that is, reduced by 99 percent. There were also dramatic devaluations of more than 80 percent for lignite. Germany is the largest lignite producer in the world. There are similar trends, even if not quite as massive, in Great Britain and Poland, for example. […] If one now assumes that coal should absorb the declines in production of natural gas and crude oil in the coming decades , an expansion of global production by 30 percent would initially be conceivable. This increase should come primarily from Australia, China, Russia, Ukraine, Kazakhstan and South Africa. Thereafter, the subsidy will remain constant, only to decrease continuously from 2025 onwards. "
In the second quarter of 2016, the world market price for steam coal was around 56 euros per tonne of coal.
Delivery rate
According to the 67th BP World Energy Report, China had a share of 46.4% of global coal production in 2017, just over double the share of all OECD countries (22.6%). The USA (9.9%), Australia (7.9%), India (7.8%), Indonesia (7.2%) and Russia (5.5%) followed far behind. Germany ranked 11th with 1.0% of world production.

and anthracite) since 1978 (in million t)

Classifications and standard quality features
General information on classification
The classification of coal or coal is carried out according to different systems, depending on the focus. From a technical point of view, two terms in particular must be carefully distinguished: types of coal and types of coal .
Types of coal
The types of coal are coal- specific, traditional names for grain sizes that are separated ( classified ) from the unsorted raw coal ( conveyed coal) by sieving . While originally each German coal mining area had its own, albeit often similar, variety denominations, a uniform variety system was introduced in 1941. According to this, all fragments that are larger than 80 mm are referred to as lump coal . The smaller components of the lump charcoal (150–80 mm) are called nibbling . Below 80 mm, a further distinction is made between
- Nut charcoal
- Nut 1, 80-50 mm
- Nut 2, 50-30 mm
- Nut 3, 30-18 mm
- Nut 4, 18-10 mm
- Nut 5, 10-6 mm
- Fine coal
- Fine coal I, 10-0 mm
- Fine coal II, 6-0 mm
- Dust charcoal, 0.5-0 mm
Types of coal
The types of coal are differentiated according to their chemical and physical properties, which are closely related to the geological history of the deposit. Mainly two criteria are used to define the type of coal: coal type and coal rank .
- The type of coal is closely linked to the sedimentation history of coal, especially to the type of deposit space and the type of organic material deposited. A distinction is made between two basic types of coal : humus coal and sapropel coal . Humus coals are coals in the strict sense of the word and the much more common of the two main types of coal. They have their origin in moist terrestrial storage areas characterized by dense vegetation (“coal swamps”), arise largely from woody and herbaceous plant matter and develop via the intermediate stage peat . Macroscopically , they are ideally characterized by a striped structure in the centimeter range. Sapropel coals, on the other hand, arise in still waters and come from non-woody material from land plants (mainly leaves ), but also partly from algae and develop from digested sludge (sapropel). They do not have a stripy structure and break like a shell . They often form thin interlockings in a succession of humus coals and testify to increasing waterlogging of the deposit area.
- The coal rank is an expression of the degree of maturity or the progress of coalification . It is closely linked to the further geological history (see → Diagenesis ) of the once deposited organic material, peat or digested sludge, especially with the amount of sinking into deeper areas of the earth's crust and the associated increase in ambient temperature . As the degree of maturity increases, the macroscopically and microscopically detectable differences between humus and sapropel coals can increasingly blur.
For a more detailed breakdown of the types of coal, see below .
Composition of coal

Coal is made up of crystalline carbon, organic matter, minerals and water. The level of the proportion of mineral (inorganic) substance is also referred to as the degree of carbon . The term “ash content” is often used because mineral substances hardly contribute to the calorific value of the coal; after the coal is burned, the coal remains and forms the ash . However, the mineral substance of unburned coal also contains so-called volatile minerals , which escape as gases during combustion and do not contribute to the formation of ash (e.g. carbon dioxide bound in carbonates ). Minerals present in unburned coal are therefore not necessarily represented in the corresponding ashes. The mineral substance can have entered the coal primarily, i.e. in the course of sedimentation (e.g. clay minerals , silt particles made of quartz ) or secondarily during diagenesis (clay minerals, calcite ). The degree of coal and ash content have a decisive influence on the value or quality of coal.
The organic matter consists mainly of carbon, hydrogen, nitrogen, sulfur and oxygen. It forms a complex macromolecular framework, the structure and composition of which varies depending on the type of coal and the origin of the coal. As the degree of coalification increases, the ratio of carbon to hydrogen, oxygen, nitrogen and sulfur shifts in the direction of carbon (see also table below), with the proportion of oxygen being particularly strong and the proportion of hydrogen decreasing slightly and the proportion of sulfur and nitrogen remaining more or less constant.
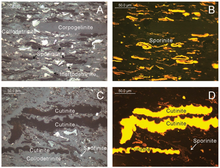
Based on their optical properties in thin and polished sections, the organic matter is divided into different macerals . The proportion of these macerals in a coal is primarily based on which plants (parts) it originated from. In coal petrography , the coal type is determined on the basis of the share ratio, and the coal rank is determined on the basis of the reflectivity especially of the vitrinite share.
Reference states
While the content of organic matter and minerals is practically invariable under normal ambient conditions, the water content can fluctuate widely, i.e. H. Coal absorbs water and gives it off again. Therefore, reference states are defined that take this into account. A distinction is mainly made between the following states:
- raw: in the condition in which it is delivered (fuel at the time of sampling without loss of water, roughly corresponds to the condition in which it is used)
- on: wet for analysis (fuel finely ground at the time of analysis)
- wf: anhydrous (fuel dried at 106 ° C)
- wafer: water and ash free (fuel dried and without ash)
The reference state waf is hypothetical, here the proportion of water and ash has been subtracted by calculation; it is used to characterize the organic matter. All reference states can be converted into one another.
Analytical methods
Since some of its properties are of considerable importance for the material value of coal (pricing based on analysis data) and must be determined nationally and internationally using the same methods, national (DIN) and international standards (ISO) have been developed for the analysis of coal . In Germany, the working committee "Testing of solid fuels" in the Mining Standards Committee (FABERG) of DIN was responsible, internationally the ISO / TC 27 "Solid Mineral Fuels" technical committee of ISO .
Determination of the water content
In terms of water content, a distinction is made between coarse and hygroscopic moisture. Coarse moisture is the purely mechanically adhering water, hygroscopic moisture is the water held in the capillaries of the grains of coal. The determination is carried out in accordance with DIN 51718. For most coals, the determination is carried out in two stages: the coarse moisture is determined in a drying cabinet at 30 ± 2 ° C, the hygroscopic moisture at 106 ± 2 ° C under a nitrogen atmosphere. In the case of oxidatively stable coals (anthracite), the total water content can also be determined in one step at 106 ° C in air. Another method is xylene distillation with subsequent volumetric water determination.
Determination of the ashes
Ash is the inorganic residue that, according to DIN 51719, remains after burning the coal in the furnace at 815 ° C. Ash is a mixture of alkali, alkaline earth, iron and aluminum salts such as oxides, sulfates, silicates and phosphates.
Determination of the volatile content
Volatile components cannot be clearly defined, the determination is a convention procedure. These are determined according to DIN 51720: the coal is heated to 900 ° C. for 7 minutes. Escaping components, corrected for the water that also escapes, are by convention the volatile components.
Types of coal
General information on the subdivision
Coal types are not divided uniformly internationally. The table shows a compilation of the Federal Institute for Geosciences and Raw Materials (BGR). The main criteria for classification are energy content (calorific value), volatile components and vitrinite reflection.
| Coal types | ||
|---|---|---|
| UN-ECE | United States | Germany |
| Peat | Peat | peat |
| Ortho-Lignite | Lignite | Soft brown coal |
| Meta-lignite | Lignite / sub-bituminous coal | Matt brown coal |
| Sub-bituminous coal | Sub-bituminous coal | Luster brown coal |
| Bitumiuous coal | Sub-bituminous coal / medium volatile bituminous coal | Flame charcoal |
| Medium volatile bituminous coal | Gas flame coal | |
| Medium volatile bituminous coal | Gas coal | |
| Medium volatile bituminous coal | Charcoal | |
| Low volatile bituminous coal | Edible charcoal | |
| Anthracite | Semi-anthracite | Lean coal |
| Anthracite | anthracite | |
A similar classification of the types of coal takes place according to the content of volatile components, this classification is especially common in Ruhr mining. The next table shows the types of coal and typical elemental compositions.
| Surname | Coalification | Water content of the raw coal | Volatiles
(waf) in% |
C carbon%
(waf) |
H hydrogen%
(waf) |
O oxygen%
(waf) |
Calorific value (waf) in
MJ / kg |
|---|---|---|---|---|---|---|---|
| Brown coal | low | 45-60 | 60-43 | 65-75 | 8.0-5.5 | 30-12 | <25–28 ♦ |
| Flame charcoal | 4-7 | 45-40 | 75-81 | 6.6-5.8 | > 9.8 | > 32 | |
| Gas flame coal | 3-6 | 40-35 | 81-85 | 5.8-5.6 | 9.8-7.3 | 33.0-34.2 | |
| Gas coal | 3-5 | 35-28 | 85-87.5 | 5.6-5.0 | 7.3-4.5 | 33.9-34.8 | |
| Charcoal | 2-4 | 28-19 | 87.5-89.5 | 5.0-4.5 | 4.5-3.2 | 34.5-35.6 | |
| Edible charcoal | 2-4 | 19-14 | 89.5-90.5 | 4.5-4.0 | 3.2-2.8 | 35.2-35.6 | |
| Lean coal | 1-3 | 14-12 | 90.5-91.5 | 4.0-3.75 | 2.8-2.5 | 35.2-35.5 | |
| anthracite | high | <2 | <12 | > 91.5 | <3.75 | <2.0 | 35.0-35.3 |
♦ Due to the high water content, the calorific value of raw lignite is only about 2/3 as high as that of hard coal.
Coal also contains nitrogen in the range from about 0.8% to about 2%, sulfur in the range from about 0.2% to about 3%, in individual cases also higher. With increasing coalification, the volatile components, the hydrogen and oxygen content decrease, the calorific value increases.
Brown coal
Today, lignite - ground and dried - is used almost exclusively as fuel to generate electricity . The proportion of production that is pressed into briquettes has decreased significantly. Lignite is brownish to black and has a high moisture content of up to 50 percent. Their carbon content is 65-70% in anhydrous coal. The sulfur content is up to 3%. It is mostly extracted in open pit mining .
There are three large lignite mining areas in Germany:
The largest German lignite company is RWE Rheinbraun AG in Cologne , its briquettes are marketed under the name Union-Brikett .
The time of origin of lignite is the Tertiary . As with hard coal, the wood from dead trees also plays a role here, as it went through the process of coalification under pressure and in the absence of air. However, lignite originated in a more recent geological age, which is why it differs qualitatively from hard coal, for example, in its higher sulfur content and a coarse, loose and porous base mass in which large inclusions (sometimes whole tree stumps) can be found.
When it comes to lignite, a distinction is made between bright lignite, matt lignite and soft lignite. The types with a high proportion of volatile components can be processed into coke in a coking plant . Depending on the temperature of the process, smoldering or Grudekoks are obtained . Lignite coke is primarily used on an industrial scale for filtration , the material replacing the activated charcoal made of wood, which is customary on a laboratory scale .
Lignite fly ash is a by-product of lignite combustion.
According to Gerhard Madaus' book on medicinal plants from 1938, coltsfoot is the only plant that can thrive on pure brown coal without any problems.
Hard coal


Hard coal is a collective term for higher quality coal. It arose from large stands of primeval forest that accumulated large amounts of biomass in the process of dying, similar to what happens in a peat bog today. These deposits were partially covered at regular intervals (which is why there are usually several coal seams in hard coal mining ) by other sediments such as clays and sand / sandstones. As a result, the organic starting material was compressed and converted under the exclusion of air and at high pressures and temperatures until a solid bond of carbon , water and non-combustible mineral inclusions was created. The mineral substance is changed during combustion and then appears in the form of ash. Hard coal is characterized by a black, solid base, in which inclusions and prints of prehistoric plants can be found.
Mining areas in Germany:
- Ruhr area
- Saarland (1429-2012)
- Ibbenbüren (anthracite coal)
- Aachen Revier (1113–1997)
- Province of Upper Silesia (1750–1945)
- Zwickau coal field (1138–1978)
- Lugau-Oelsnitzer coal field (1844–1971)
- Döhlen Basin (1542–1967)
In Germany, hard coal is extracted in mines up to 1,750 meters deep . It is extracted either with a coal plow or a shearer loader . In Colombia, South Africa or Australia, coal can be mined more cheaply than in Germany. This is why imported coal is used more frequently than domestic coal in German power plants. So that only coal from abroad is not converted into electricity in Germany, there is a hard coal subsidy that expires in 2018.
Other terms
Pitch coal
Pitch coal was mined in Bavaria (Penzberg, Peißenberg, Peiting, Hausham etc.). Their age corresponds to that of lignite. Due to the higher mountain pressure, however, it has properties like anthracite. The tunnels in the mining department of the Deutsches Museum in Munich were built with pitch coal on the walls.
Shungite charcoal
Rocks found in some places in Finland and Russia that are up to 95% carbon are known as shungite coal.
Sapropel coal
In various hard coal deposits there is a very small amount of sapropel coal, which was formed from digested sludge. It is soft and easy to edit, so that z. B. Figures can be carved.
use
Coal is mainly used as a solid fuel to generate heat through combustion. To z. B. to generate electrical energy in coal-fired power plants , water vapor is generated by means of the released heat, which in turn drives steam turbines . In order to compare which amount of energy can be obtained with which coal, one usually uses the hard coal unit .
In 2003, 24.4% of primary energy and 40.1% of electrical energy worldwide were generated through the industrial use of coal as fuel. Hard coal and lignite are roughly equally represented. Various techniques are used in modern coal-fired power plants to reduce pollutants and increase efficiency.
A considerable part of the coal after appropriate treatment for the reduction of ores , mainly iron ore in blast furnaces used and is an important element in the process of iron smelting .
In Great Britain , coal mining developed from the beginning of the 18th century into an essential basis for the industrialization that started there . From the 19th century, the coal was also used to produce town gas , which was used for street lighting, cooking and heating. In gasworks , town gas was obtained from coal using dry distillation - coke was a by-product . In the 20th century, town gas was largely replaced by natural gas .
In the 18th century, brown coal was used as a color pigment under the name Umber or Cölln earth .
Products made from coal
coke
Coke is a solid, carbonaceous residue that is preferably obtained from low-ash and low-sulfur fatty coal. In coking plants, the volatile components are removed by heating them in an oven with the exclusion of air at more than 1000 ° C, so that the solid carbon and the remaining ash melt together. Coke oven gas and coal tar are produced as by-products . This process, known as coking, is one of the processes used to refine coal . Coke burns with an almost invisible blue flame. There is no soot or visible smoke gas. Coke is used as fuel and as a reducing agent in iron production in blast furnaces. It has a dull gray color and is hard and porous.
Briquettes
Briquettes are made by pressing, mostly from lignite. Hard coal briquettes (egg coals) are of little importance for house fires in Germany, only those made from anthracite are still sold.
Coal liquefaction
Coal liquefaction describes chemical processes that produce liquid hydrocarbons from solid coal. The process of direct hydrogenation of coal was used to produce gases, carburetor and diesel fuels. The motivation for the large-scale use of coal liquefaction is the replacement of crude oil as a raw material for the petrochemical and energy sectors. The processes become more important when petroleum is not available in sufficient quantities.
Environmental and climate issues
Extraction

The extraction of coal in open-cast mining is associated with an immense amount of land . While in Germany only lignite is mined in open pit mining, in other countries z. Sometimes hard coal is mined in this way, for example in the El Cerrejón mine in Colombia, the largest hard coal mine in South America with an area of around 690 km².
Another form is the v. a. Mountaintop removal mining practiced in the American Appalachian Mountains , in which mountain tops are first blasted and removed in order to then be able to extract the coal in open-cast mining. In the Appalachian Mountains alone, the mining areas currently (2012) extend over an area of around 5,700 square kilometers, often original forest areas. In addition, heavy metals such as arsenic and mercury are released during mining, which pollute the environment and endanger the health of local residents, and floods often occur, as rivers are often buried through the deposition of overburden in valleys.
In order to be able to exploit deposits as fully as possible, entire villages are sometimes relocated, which can lead to potential for conflict with the population (see also list of excavated towns ). Under certain circumstances, ecologically or culturally particularly valuable areas are also destroyed - examples of this are the Lacoma pond landscape and the village of Lakoma , which had to give way to the Cottbus-Nord opencast mine , as well as numerous villages in the Sorbian settlement area that had to give way in recent decades or, in some cases, until today are threatened (see Mühlrose ).
In lignite opencast mines, similar to wide, dry fields in agriculture, large amounts of dust can arise. Therefore, the use of efficient dust control technology is essential.
Another aspect is the lowering of the groundwater level to a level below the deepest extraction level in open-cast lignite mining. This is done with submersible pumps in specially created wells. A lowering of the groundwater level can have negative effects on the flora , as the upper soil layers can dry out. The lowering also leads to the drying out of nearby wells, which draw their water from the affected aquifer .
Conversely, the closure of an opencast mine leads to an increase in the groundwater level as soon as the submersible pumps are switched off. This can cause major damage to the buildings in the area. A well-known example of this is the area around the town of Korschenbroich , whose residents have been struggling with the rising groundwater level since the Garzweiler I opencast mine was gradually closed.
The greenhouse gas methane also escapes from coal mines .
combustion
When coal is burned, carbon dioxide , water vapor and other gases such as sulfur dioxide are produced , and pollutants such as fly ash , fine dust and heavy metals are also emitted which are harmful to the environment and health . Compared to other fossil fuels used in large quantities, the largest amount of the greenhouse gas carbon dioxide (CO 2 ) is released per usable energy content . Because of their lower efficiency, lignite power plants (approx. 1080 grams CO 2 / kWh ) are less favorable than hard coal power plants (approx. 800 g CO 2 / kWh). The release of CO 2 during combustion with oxygen can only be reduced by improving the efficiency of the power plants and thus lowering the consumption of coal. In addition to the CO 2 emitted directly in power plants and industrial plants, further CO 2 can be released through unwanted coal fires .
The sulfur dioxide , which is mainly produced when burning lignite , is partly responsible for acid rain . In modern hard coal and lignite power plants, the exhaust gases are cleaned of sulfur dioxide in flue gas desulphurization systems (see also FGD gypsum ), nitrogen oxides by catalytic ( SCR ) or non-catalytic ( SNCR ) denitrification and dust in electrical separators. The ash produced when coal is burned contains increased concentrations of heavy metals such as B. arsenic and mercury , but also the radioactive elements uranium and thorium . Due to the emission of dust, particularly in countries with only low environmental protection regulations, there is severe, health-endangering air pollution and smog . This is a big problem, especially in China, which relies heavily on coal as an energy source.
Environmental, health and climate damage are negative effects of the use of coal that are not borne by the polluters, but by the general public. In economic terms, one speaks of so-called external costs . These social and ecological costs of conventional energy generation have a significant impact and in some cases even exceed the end customer prices of electricity consumers. For the USA, for example, external costs of generating electricity from coal were determined to be between 175 and 523 billion US dollars per year, with a conservatively calculated probable mean of 345 billion dollars or 17.8 ct / kWh. Some negative consequential effects such as B. Environmental effects due to the release of toxic chemicals and heavy metals into the environment, eutrophication of waters due to nitrogen input, secondary effects of acid rain and some of the consequences of global warming.
Coal exit
In order to minimize the environmental, health and climate damage caused by energy generation and to still be able to meet the two-degree target , an exit from the generation of electricity from coal is seen as necessary. If the two-degree target is to be achieved with a probability of more than 50%, according to data from the IPCC, a maximum of between 870 and 1,240 gigatons (billion tons) of carbon dioxide may be released between 2011 and 2050 . Converted to the reserves, this means that in the global context around a third of the oil reserves, half of the natural gas reserves and more than 80% of the coal reserves must not be burned. A central element of the energy transition is therefore a coal exit.
The strong increase in the expansion of coal-fired power plants up until a few years ago has recently lost momentum; since 2010 only a third of the planned coal-fired power plants have actually been implemented worldwide. In China and the USA, coal consumption is falling and is decoupling from economic growth. Several governments are aiming for a significant reduction or a complete phase-out of coal. The Canadian province of Ontario was the first major administrative unit to phase out coal by 2014. In order to reduce CO 2 emissions and smog, 12 out of 34 Chinese provinces want to reduce their coal consumption.
See also
literature
- 2007, web.mit.edu: The Future of Coal. Options for a Carbon-Constrained World Interdisciplinary Study from the Massachusetts Institute of Technology (MIT), ISBN 978-0-615-14092-6
- January 2009: Science Practice - Coal . Special issue , Aulis-Verlag, series Praxis der Naturwissenschaften - Chemie in der Schule (1/58) → gvst.de
- June 2, 2015: Heinrich Böll Foundation in cooperation with BUND , boell.de: Kohleatlas
Web links
Individual evidence
- ↑ Special brown coal. Archived from the original on January 12, 2012 ; Retrieved March 3, 2015 .
- ↑ a b Website of the Energy Watch Group : The range of coal is clearly overestimated ( Memento from January 28, 2016 in the Internet Archive ) (April 3, 2007; PDF; 146 kB), last accessed on January 28, 2016.
- ↑ David Hibbett, Robert Blanchette, Paul Kenrick, Benjamin Mills: Climate, decay, and the death of the coal forests . In: Current Biology . tape 26 , no. 13 , 2016, p. R563-567 , doi : 10.1016 / j.cub.2016.01.014 (alternative full text access : Clark University ).
- ^ Hans Murawski, Wilhelm Meyer: Geological dictionary. 12th edition. Spektrum Akademischer Verlag, 2010, ISBN 978-3-8274-1810-4 , pp. 97, 121.
- ↑ Dimitrios Floudas, Manfred Binder, Robert Riley and 68 other authors: The Paleozoic Origin of Enzymatic Lignin Decomposition Reconstructed from 31 Fungal Genomes . In: Science . tape 336 , no. 6089 , 2012, p. 1715–1719 , doi : 10.1126 / science.1221748 (alternative full text access: Clark University ).
- ^ Nicola Armaroli , Vincenzo Balzani : Energy for a Sustainable World - From the Oil Age to a Sun-Powered Future . Wiley-VCH 2011, p. 86.
- ↑ Brown coal in Germany 2013. Bundesverband Braunkohle, accessed on March 6, 2015 .
- ↑ Third country coal price. Federal Office of Economics and Export Control , accessed on November 7, 2016 .
- ↑ BP plc: BP Statistical Review of World Energy 2018. London, 2018 ( PDF 6.5 MB), p. 38
- ↑ a b BGR: Energy Study 2015. Reserves, resources and availability of energy raw materials (19). Hanover 2015 ( online )
- ^ Wilhelm Gumz, Lothar Hardt: Short manual of fuel and combustion technology. Springer-Verlag, 1962, ISBN 978-3-642-51615-3 , p. 203 (Table 5-9)
- ↑ a b c coal. Spectrum online encyclopedia of geosciences
- ↑ a b Jennifer MK O'Keefe, Achim Bechtel, Kimon Christanis and 10 other authors: On the fundamental difference between coal rank and coal type. International Journal of Coal Geology. Vol. 118, 2013, pp. 58–87, doi: 10.1016 / j.coal.2013.08.007 (alternative full text access : Smithsonian Libraries )
- ↑ Sapropel coal. Spectrum online encyclopedia of geosciences
- ↑ Eberhard Lindner; Chemistry for engineers; Lindner Verlag Karlsruhe, p. 258.
- ↑ Energy resources 2009: Reserves, resources, availability, Federal Institute for Geosciences and Raw Materials (BGR), Hanover, page 21, ISBN 978-3-9813373-1-0 .
- ↑ International classification of coal types; PDF file (149 kB).
- ↑ Franck and Knoop: Coal Refinement. Chemistry and Technology, Springer-Verlag, Heidelberg; after G.Kölling and F.Schnur: Chemical raw materials from coal, Thieme, Stuttgart 1977.
- ↑ Away with the mountains . In: Die Zeit , October 18, 2007. Retrieved May 16, 2012.
- ↑ Josh Gabbatiss: Coal mines emit more methane than oil-and-gas sector, study finds. Carbon Brief, March 24, 2020, accessed on March 29, 2020 .
- ↑ Die Zeit, Beijing - How sick does smog make you? , February 27, 2014.
- ↑ International Energy Agency: CO2 Emissions from Fuel Combustion 2010 - Highlights (PDF; 2.3 MB). P. 37.
- ↑ Konrad Kleinknecht : Who sits in the greenhouse , Piper, 2007, ISBN 978-3-492-05011-1 .
- ↑ COAL ASH IN ALASKA: OUR HEALTH, OUR RIGHT TO KNOW - A Report on Toxic Chemicals Found in Coal Combustion Waste in Alaska ( Memento from July 18, 2014 in the Internet Archive ). P. 8.
- ↑ a b Deutschlandfunk, coal power: smog country China as a climate saver , April 11, 2014.
- ↑ G20: Coal power plant subsidies more than doubled - derStandard.at. In: DerStandard.at. June 26, 2019, accessed on November 22, 2019 (Austrian German).
- ^ Nicola Armaroli , Vincenzo Balzani , Towards an electricity-powered world . In: Energy and Environmental Science 4, (2011), 3193-3222, p. 3195, doi : 10.1039 / c1ee01249e .
- ^ Epstein et al., Full cost accounting for the life cycle of coal . In: Annals of the New York Academy of Sciences 1219, (2011), 73-98, p. 93, doi : 10.1111 / j.1749-6632.2010.05890.x .
- ^ IPCC, working group three published results: Summary for Policymakers , April 13, 2014.
- ↑ Christophe McGlade, Paul Ekins, The geographical distribution of fossil fuels unused When limiting global warming to 2 ° C . Nature 517, (2015), 187-190, doi : 10.1038 / nature14016 .
- ^ Germanwatch: Evidence for a turnaround in international climate and energy policy. Bonn 2015, PDF ( Memento from March 8, 2016 in the Internet Archive )
- ^ Province of Ontario, press release, Creating Cleaner Air in Ontario - Province Has Eliminated Coal-Fired Generation , April 15, 2014.







