Thorium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Thorium, Th, 90 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Actinoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | Ac , 7 , f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-29-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-139-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.308 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 11 ppm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 232.0377 (4) and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 180 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 206 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Rn ] 6 d 2 7 s 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.30670 (25) eV ≈ 608.5 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 12.10 (20) eV ≈ 1 167 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 18th.32 (5) eV ≈ 1 768 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 28.648 (25) eV ≈ 2 764.1 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 58.0 (1.9) eV ≈ 5 600 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | Cubic area-centered | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 11.724 g / cm 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 3.0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 8.4 · 10 −5 ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2028 K (1755 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 5061 K (4788 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 19.80 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 530 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 16 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2490 m s −1 at 293.15 K. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 6.67 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 54 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 4,3,2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.3 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Thorium (after the Germanic god Thor ) is a chemical element with the element symbol Th and the atomic number 90. In the periodic table it is in the group of actinides ( 7th period , f-block ).
history
Hans Morten Thrane Esmark found a black mineral in 1828 on the Norwegian island of Løvøya (Løvø), near the village of Brevik in the Langesundsfjord . He gave this sample to his father, Jens Esmark, a leading Norwegian professor of geology. Esmark could not assign this sample to any previously known mineral and sent the sample, in which he suspected an unknown substance, to the Swedish chemist Jöns Jakob Berzelius . In the same year he discovered that this mineral ( thorite ) consisted of almost 60% of a new oxide ( thorium dioxide ). He named the metal on which the oxide was based, Thorium, after the god Thor . Berzelius published the discovery of the new mineral in 1829.
Berzelius already owned a rock sample in 1815, which he believed to be a new mineral. He assigned this mineral to a new oxide and named the corresponding metal Thor after the Germanic god of thunder . In 1824, however, it turned out that this supposedly new mineral was xenotime ( yttrium phosphate ).
In 1898, Marie Curie and Gerhard Schmidt (1865–1949) discovered the radioactivity of thorium at the same time .
In 1914, Lely and Hamburger succeeded in producing pure metal for the first time.
Occurrence
Thorium compounds are often found in monazite sands ( Ce , La , Nd , Th) [ PO 4 ] + 4 ... 12% ThO 2 , in the mineral, which is isomorphic with zirconium, thorite ThSiO 4 and in thorianite (Th, U) O 2 . Also titanite and zircon themselves contain smaller amounts of thorium.
Thorium occurs in the earth's crust at a frequency of 7 to 13 mg per kg; so it is twice to three times as common as uranium . In general, due to its lithophilic character , the element is represented in small quantities in almost all silicate rocks.
The coal used annually for power generation worldwide contains around 10,000 t of uranium and 25,000 t of thorium, which either end up in the environment or accumulate in power plant ash and filter dust.
The radioactive metal is mined in Australia , Norway , Sri Lanka , Canada , USA , India , Lapland and Brazil . Silent deposits of around 800,000 tons are located in Turkey, mainly in the Eskişehir province in the Sivrihisar district . Human bones contain between 2 and 12 µg thorium per kg bone mass. Between 0.05 and 3 µg are absorbed daily through food and water.
properties
Pure thorium is a silver-white metal that is stable in air at room temperature and retains its shine for a few months. If it is soiled with its oxide, it slowly tarnishes in the air and turns gray and finally black.
The physical properties of thorium are highly dependent on its oxide pollution. Many “pure” varieties often contain a few per thousand thorium dioxide. However, high-purity thorium is also available. Pure thorium is soft and very ductile, it can be cold rolled and drawn.
Thorium is polymorphic with two known modifications. At over 1400 ° C, it changes from a face-centered cubic to a body-centered cubic structure.
Thorium is only attacked very slowly by water; it dissolves only slowly in most dilute acids (hydrofluoric, nitric, sulfuric acid) and in concentrated hydrochloric and phosphoric acid. It dissolves well in smoking nitric acid and aqua regia . Powdered thorium has a pyrophoric effect when finely divided . Thorium burns in air with a white, brightly shining flame.
presentation
Ore processing
Thorium occurs in primary and secondary deposits. In ore processing , the ores from the primary deposit are crushed and ground. The enrichment is usually done by flotation . Accompanying alkaline earth carbonates are dissolved by a hydrochloric acid treatment. In the case of secondary deposits , gravity separation of the mineral fractions is usually carried out first, followed by magnetic separation . The monazite can be separated from the ferromagnetic and non-magnetic minerals by its paramagnetism .
A concentrate of thorium and rare earth compounds is then produced through thickening, filtration and calcination .
Ore exploration and thorium extraction
Monazite is a relatively inert mineral . The simplest method is digestion with hot sulfuric acid at over 200 ° C with subsequent precipitation by dilution with water. The problems of the process are the slow dissolution of the grains as well as the complexation of the dissolved metal ions by phosphates and sulfates and the associated small process window. For this reason, an alkaline digestion with hot sodium hydroxide solution was developed, which allows the phosphate ions to be separated off. However, this process did not prevail.
From around 1950, interest in higher purity thorium (nuclear grade) increased. This led to the expansion of the sulfuric acid process by precipitation with oxalates , which are then converted to thorium hydroxide. This is still contaminated with rare earths. Therefore, the hydroxides were dissolved with nitric acid in the form of nitrates . The thorium was extracted from the solution by means of solvent extraction - tri-n-butyl-phosphate (TBP) in kerosene , for how it works, see also the PUREX process .
reduction
Since thorium has a low electronegativity may direct reduction not by means of its connections carbon or hydrogen done, there would be z. B. high-melting thorium carbides or - form hydrides .
One possibility is the electrolysis of thorium halides in molten salts. B .:
- KThF 5 in NaCl
- ThF 4 in NaCl - KCl
- ThCl 4 in NaCl - KCl
or the implementation with base metals:
- ThO 2 with approx
- ThCl 4 with Mg ( Kroll process )
- ThF 4 with approx
or via a gas phase transport:
- thermal decomposition of ThI 4 ( Van-Arkel-de-Boer process )
The powder or metal sponge obtained in this way is remelted into solid material under protective gas or in a vacuum.
Isotopes and decay series
Almost only the isotope with the longest half-life 232 Th occurs in nature . Thorium contributes to geothermal energy through its decay . Because 232 Th was long thought to be the beginning of one of the naturally occurring series of decays, it was named after him. The decay products of naturally occurring thorium-232 are in the following order:
- Radium 228 Ra ( half-life 5.75 a),
- Actinium 228 Ac (6.15 h),
- Thorium 228 Th (1.9116 a),
- Radium 224 Ra (3.66 d),
- Radon 220 Rn (55.6 s),
- Polonium 216 Po (0.145 s),
- Lead 212 Pb (10.6 h),
- Bismuth 212 Bi (60.55 min),
- from it 64% Polonium 212 Po (3 · 10 −7 s) and
- to 36% thallium 208 Tl (3.053 min),
- from both stable lead 208 Pb.
For the complete decay series up to its beginning see: Thorium series .
use
Incandescent light
Thorium in the form of its oxide was used to make incandescent mantles . These mantles were made by soaking fabric with a solution of 99% thorium nitrate and 1% cerium nitrate . When ignited for the first time, the organic tissue was burned and the thorium nitrate decomposed into thorium dioxide and nitrous gases. This left a fragile structure that gave off a white light in the gas flame. This glow had nothing to do with the very weak radioactivity of the thorium, but is an ordinary glow from the heat of the gas flame. Due to the radioactivity, other materials have now been used.
Nuclear energy
Thorium can be used to produce the fissile uranium isotope 233 U. In contrast to the uranium-plutonium breeder reactor (the fast breeder ), this is also possible in a reactor in which the nuclear fission takes place using thermal neutrons. This is due to the particularly high cross-section of 232 Th for capturing a thermal neutron. The achievable breeding rates are lower with such a thermal breeder than with the fast breeder.
233 Th is hatched from thorium 232 Th through neutron radiation ; this breaks down into uranium 233 U via protactinium 233 Pa .
- The times given are half-lives .
Tests with thorium in MOX fuel elements had already been carried out in Lingen in the 1970s . The Shippingport light water reactor was in operation as a thermal breeder from 1977 to 1982. The early high temperature reactors (HTR) with thorium use, e.g. B. the THTR-300 , hatched fewer 233 U than they consumed of fissile material, so they were not breeder reactors. Only about 4% of the thorium inventory could be used to generate energy. In addition to the addition of thorium, these HTRs were dependent on a constant supply of fission material in a highly enriched, weapon-grade form (93% 235 U), which soon turned out to be unacceptable for reasons of proliferation security, so that newer HTR concepts are based on the classic U / Pu cycle with low enrichment Uranium, d. H. without thorium, concentrate. The German THTR-300 was shut down in 1989 after 423 days of full load operation and many problems. In 2002 tests with thorium took place in Obrigheim . A new five-year series of tests on the use of thorium in MOX fuel elements has been running since April 2013 in the Norwegian research reactor Halden . The aim is to use the process in commercial nuclear power plants and also to mine the plutonium . The molten salt reactor should be mentioned as a current concept for a thermal breeder based on thorium . Such a thermal breeder shows safety problems; therefore the concept of a fast molten salt breeder is discussed. The concept of the accelerator- driven Rubbiatron reactor is also based on thorium.
Since thorium is present in greater quantities than uranium, it could potentially be an important source of energy in the future following the expected decline in global uranium supplies. In the Anglo-Saxon region in particular, there was an intensive campaign in the early 2010s for the use of thorium to allegedly solve almost all energy problems. Critics of this campaign speak of thorium hype or even astroturfing . Studies for the Norwegian and UK governments warn of high expectations regarding thorium use. More recent studies also indicate that a nuclear technology involving thorium harbors considerable proliferation risks. Another safety disadvantage of the use of thorium is that the fission of uranium-233 produces around 60% fewer delayed neutrons than the fission of uranium-235; this increases the risk of criticality accidents .
Research on the use of thorium in nuclear power plants is currently being carried out in India in particular , as this country has the world's largest deposits of thorium. The completion of the Prototype Fast Breeder Reactor (prototype fast breeder reactor, PFBR ) has been delayed for years. This PFBR is said to have an output of 500 MW, to work with plutonium as fissile material and to contain thorium in the breeding mantle, which is converted into 233 U for other applications.
The whistleblower Rainer Moormann published a critical statement on the use of thorium in 2018 and pointed out, above all, the increased risk of proliferation due to the fact that an atomic bomb made of 233 U could easily be built, even for terrorists .
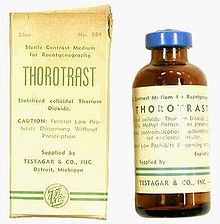
Thorotrast
A stabilized suspension of colloidal thorium dioxide was used as an X-ray contrast medium for angiography from 1931 under this trade name until the late 1940s . However, it accumulates in the reticulohistiocytic system and can lead to cancer due to locally increased radiation exposure. There are clear associations between Thorotrast and biliary carcinoma ; In addition, an angiosarcoma of the liver, an otherwise very rare malignant tumor of the liver, can be induced by Thorotrast. Carcinomas of the paranasal sinuses after administration of Thorotrast have been described. Typically, the disease occurs 30–35 years after exposure.
Instead of Thorotrast, barium sulfate and significantly improved aromatic iodine derivatives are used as X-ray contrast media today.
Other uses
To improve the ignition properties of the electrodes used in tungsten inert gas welding (TIG welding), thorium dioxide in the order of 1 to 4% was added. This use has now almost been discontinued due to the radiation exposure from vapors and grinding dust. Modern TIG electrodes work with cerium additives.
As Glühelektrodenwerkstoff inserted tungsten wire is to reduce the electron work function doped with about 1-3% thoria. This makes it possible to reduce the temperature in electron tubes necessary for a comparable emission and improves the starting behavior of discharge lamps . In lamp construction, thorium is also used as a getter in the form of thorium dioxide pills or thorium foil.
Thorium dioxide was added to the glass for high-quality optical lenses in order to produce lenses with a very high optical refractive index with a small optical dispersion . Optical devices from the time of World War II (e.g. the Aero-Ektar from Kodak ) or the early post-war years (e.g. some Summicron lenses from Leitz ) occasionally contain thorium glass . Lenses containing thorium have a slight yellow tinge that intensifies over time, which can be at least partially removed by intensive exposure to UV light . Because of the radiation emitted by the thorium, glass containing thorium is no longer commercially produced today. Glasses containing lanthanum (e.g. LaK9) can replace thorium glass.
The isotope Th-229 has the unique property that its atomic nucleus has an excited state ( nuclear isomer ) only 8.28 eV above the ground state (new measurements by LMU Munich, September 2019; ENSDF data come from 1994 and 2007). This corresponds nm ultraviolet light with wavelengths of 149.7. Therefore, it could be possible to excite this state with laser light and a nuclear core clock to construct. For this purpose, a research project in which the PTB , the Technical University of Vienna , the University of Delaware , the Ludwig Maximilians University , the Max Planck Institute for Nuclear Physics and the Fraunhofer Institute for Laser Technology are involved, is supported by the ERC with 13, 7 million euros funded.
safety instructions
Classifications according to the CLP regulation are not available because they only include the chemical hazard, which plays a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
Chemical toxicity
The acute chemical toxicity of thorium is estimated to be low and is largely attributed to radioactivity. This is due to the poor solubility in water of 0.0001 μg per liter of the pure metal as well as the most common thorium dioxide. Thorium only dissolves better in a very acidic environment from a pH value of 4. Also oxalates and other complexing agents increase the water solubility.
Radiotoxicity
The thorium isotope 232 Th, with its half-life of 14.05 billion years, is much weaker radioactive (lower dose rate) than uranium- 238, because the longer half-life means fewer decays per second and the concentration of short-lived decay products also remains lower. Thorium is both an α-emitter and a γ-emitter and due to this type of radiation is dangerous for inhalation and ingestion . Metal dusts and above all oxides are particularly dangerous in terms of radiotoxicity due to their lung penetration and can cause cancer. When storing and handling thorium and its compounds, the permanent presence of the elements from the decay series must also be taken into account. Strong beta rays and those with a high 2.6 MeV content, very energetic and penetrable gamma rays, are particularly dangerous . Furthermore, as a result of an alpha decay , the radon isotope 220 Rn, also known as thoron, is formed in the decay series , which in turn decays into polonium-216 and lead-212 in an alpha decay. With the same activity concentration, the thoron-derived products result in a 14-fold higher radiation exposure than the secondary products of 222 Rn.
Thorium compounds
In accordance with its position in the periodic table , thorium normally occurs in its compounds in the +4 oxidation state; Thorium (III) and thorium (II) compounds are less common. The carbides of the actinides without fixed stoichiometry are a specialty .
- Thorium, thorium (IV) oxide (ThO 2 ) has to 3300 ° C one of the highest melting points of all metal oxides . Only a few metals, such as tungsten , and some compounds, such as tantalum carbide , have higher melting points.
- Thorium nitrate , thorium (IV) nitrate (Th (NO 3 ) 4 ) is a colorless compound that is easily soluble in water and alcohol. Nitrate is an important intermediate product in the production of thorium (IV) oxide and thorium metal and is also used in the production of gas incandescent bodies.
- Thorium nitride, thorium (IV) nitride (Th 3 N 4 ) is produced when thorium is glowed in a nitrogen atmosphere and has a brass-colored sheen. Thorium nitride is hygroscopic and decomposes within a few hours due to humidity.
- Thorium carbide , ThC 2 forms yellow, monoclinic crystals with a melting point of 2655 ° C. The carbide becomes superconducting at around 9K. In the form of the mixed carbide (Th, U) C 2 , thorium carbide is used as a fuel in gas-cooled high-temperature reactors. The carbide mixture is represented by the reaction of thorium and uranium oxides with carbon at 1600 to 2000 ° C.
- Thorium (IV) chloride , is a white, hygroscopic , crystalline solid. Berzelius produced metallic thorium for the first time by reducing it with potassium .
Historical names
"Thorium-G"
When as a doomsday device titled "cobalt thorium G " bomb in Stanley Kubrick's film Dr. Strange or: How I learned to love the bomb , it's primarily a cobalt bomb . If thorium is used in the bomb design (possibly instead of uranium in the fission stage or in the mantle), the explosion may produce a. radioactive , toxic and long-lived Protactinium-231 , which would significantly increase the potential for contamination of the fallout . The half-life of Protactinium-231, however, is 32760 years and thus deviates significantly from the one mentioned in the film (93.7 or 100 years).
"Thorium-X"
In the first half of the 20th century, different solutions containing thorium and other radioactive nuclides were traded under the name Thorium-X . In the USA z. B. a tincture of this name was used in the radiotherapy of skin diseases until around 1960 . In Germany around 1930 there were bath additives and eczema ointments of the brand "Thorium-X", which were taken out of the market shortly afterwards due to the obvious health risks. There was also a toothpaste containing thorium X called Doramad . In addition, in the 1960s at the Münster University Clinic ( Hüfferstiftung ), Thorium-X was used in Bechterew's disease patients to prevent further stiffening of the spine. The patient received a thorium X injection once a week during an inpatient stay of around three months. The progressive stiffening was largely stopped for about 15 years.
"Ionium"
As Ionium the isotope was designated 230-Th in nuclear physics. In age dating , the term Ionium method is still used for 230-Th / 232-Th dating.
literature
- Mathias S. Wickleder, Blandine Fourest, Peter K. Dorhout: Thorium . In: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements . Springer Netherlands, Dordrecht 2008, ISBN 978-1-4020-3555-5 , Thorium, p. 52-160 , doi : 10.1007 / 1-4020-3598-5_3 ( radchem.nevada.edu [PDF]).
- Robert J. Schwankner, Alexander Brummeisl, Christian Feigl, Peter Schöffl: Early history of use of thorium. In: Geosciences. 12, 3, 1994, pp. 66-73. doi: 10.2312 / geosciences . 1994.12.66 .
Web links
- Entry to thorium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
- Seth H. Grae: Thorium , Chemical & Engineering News, 2003
- International Thorium Energy Organization - www.IThEO.org
- USA: Use of thorium aims to reduce radioactive waste
- Thorium: Human Health Fact Sheet ( Memento of 9 February 2012 at the Internet Archive ) (engl.) (PDF, 49 kB)
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (Infobox) are taken from webelements.com (Thorium) , unless otherwise stated .
- ^ IUPAC, Standard Atomic Weights Revised v2 ( Memento of March 3, 2016 in the Internet Archive ).
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on thorium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on thorium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ↑ a b c Entry for CAS no. 7440-29-1 in the GESTIS substance database of the IFA , accessed on April 5, 2008(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Magnetic Susceptibility of the Elements and Inorganic Compounds, pp. 4-147. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ Varga et al .: Determination of the 229th half-life In: Phys. Rev. C . 89, 064310 (2014); doi: 10.1103 / PhysRevC.89.064310 .
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ^ The Elements: Names and Origins - OZ. on: bbc.co.uk , accessed December 11, 2007.
- ^ A b Thorium - History & Etymology. at: elements.vanderkrogt.net , accessed December 11, 2007.
- ↑ JJ Berzelius: Investigation of a new mineral and a previously unknown earth contained therein. In: Annals of Physics and Chemistry . 92, 1829, pp. 385-415; doi: 10.1002 / andp.18290920702 .
- ^ MS Curie: Classic of science - radioactive substances by Madame Curie. In: Science News Letter . 14, 1928, pp. 137-138.
- ↑ L. Badash: The discovery of thorium's radioactivity. In: The Journal of Chemical Education . 43, 1966, pp. 219-220.
- ↑ JB Hedrick: Thorium. 1999. (PDF; 36 kB)
- ↑ AE van Arkel, JH de Boer: Representation of pure titanium, zirconium, hafnium and thorium metal. In: Journal of Inorganic and General Chemistry . 148 (1), 1925, pp. 345-350; doi: 10.1002 / zaac.19251480133 .
- ↑ D. Lely Jr., L. Hamburger: Manufacture of the elements thorium, uranium, zirconium and titanium. In: Journal of Inorganic and General Chemistry . 87 (1), 1914, pp. 209-228; doi: 10.1002 / zaac.19140870114 .
- ↑ a b B. Merkel, G. Dudel et al.: Investigations into the radiological emission of uranium tailings Schneckenstein. ( Memento from June 11, 2007 in the Internet Archive ) (PDF; 4.0 MB), TU Bergakademie Freiberg and TU Dresden 1988.
- ↑ world-nuclear.org: Naturally Occurring Radioactive Materials NORM .
- ^ J. Emsley: The Elements. Clarendon Press, Oxford 1992.
- ↑ DJ Crouse, KB Brown: Recovery of Thorium, Uranium and Rare Earths from Monazite sulfate liquors by the Amin Extraction (Amex) Process. ( Memento of the original from December 8, 2015 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (PDF). Oak Ridge National Laboratory, 1959.
- ↑ Taishi Kobayashi, Takayuki Sasaki, Ikuji Tagaki, Hirotake Moriyama: Solubility of Thorium (IV) in the Presence of Oxalic and Malonic Acids.] In: Journal of Nuclear Science and Technology. Volume 46, Issue 11, 2009. doi : 10.1080 / 18811248.2009.9711619
- ↑ Enver Oktaya, Ahmet Yaylib: Physical properties of thorium oxalate powders and their influence on the thermal decomposition. In: Journal of Nuclear Materials. Volume 288, Issue 1, January 2001, pp. 76-82.
- ↑ English page with pictures for purification in the laboratory.
- ^ Ullmann's Encyclopedia of industrial chemistry. 8th edition. 2015.
- ^ NEA, OECD: Advanced Reactors With Innovative Fuels: Second Workshop Proceedings 2002. P. 227 ff. ( Limited preview in the Google book search).
- ↑ Thorium. In: world-nuclear.org. Retrieved January 3, 2015 .
- ↑ S. Peggs et al.: Thorium Energy Futures. 2012. PDF.
- ↑ Thorium test begins. At: world-nuclear.org.
- ↑ Kun Chen from Chinese Academy of Sciences on China Thorium Molten Salt Reactor TMSR Program on YouTube .
- ↑ Thorium as a nuclear fuel. At: world-nuclear.org.
- ^ R. Martin: Super-Fuel: Thorium, the Green Energy Source for the Future. 2012. ( About the Book Super-Fuel by Richard Martin. ( Memento from May 12, 2012 in the Internet Archive ) At: superfuelbook.com. )
- ↑ Is Thorium the Biggest Energy Breakthrough Since Fire? Possibly. At: forbes.com.
- ↑ Noel Wauchope: Don't believe thorium nuclear reactor hype. In: independentaustralia.net. January 27, 2013, accessed December 13, 2015 .
- ↑ Thorium Report 2008. Oslo ( regjeringen.no PDF).
- ↑ Comparison of thorium and uranium fuel cycles. 2012. ( decc.gov.uk PDF).
- ↑ Stephen F. Ashley, Geoffrey T. Parks, William J. Nuttall, Colin Boxall, Robin W. Grimes: Thorium fuel has risks . In: Nature . tape 492 , no. 7427 , December 6, 2012, p. 31-33 , doi : 10.1038 / 492031a .
- ↑ fissilematerials.org (PDF).
- ^ India Delays Completion of its Indigenous Nuclear Reactor , accessed February 22, 2017.
- ↑ strahlentelex.de
- ↑ wiseinternational.org
- ↑ Thorium in camera lenses ( Memento of the original from January 30, 2013 in the Internet Archive ) Info: The archive link was automatically inserted and not yet checked. Please check the original and archive link according to the instructions and then remove this notice. (engl.)
- ↑ Summary of relevant literature in a contribution by user “Ill” in the Leica User Forum Permalink ( memento of the original from January 19, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (accessed April 6, 2011).
- ↑ Nadja Podbregar: First step towards the atomic nuclear clock. In: scinexx.de. September 13, 2019, accessed September 18, 2019 .
- ↑ Millions in funding for a watch project. June 20, 2017. Retrieved November 6, 2019 .
- ^ National Nuclear Data Center .
- ↑ LEIFI physics .
- ↑ Imke Frischmuth: At last precise measurement of radioactive thoron possible. ( Memento of the original from March 25, 2016 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. At: PTB.de. February 28, 2011, accessed April 7, 2016.
- ^ BK Sharma: Nuclear and Radiation Chemistry . S. 158 ( limited preview in Google Book search).


![{\ mathrm {^ {{232}} _ {{\ 90}} Th \ + \ _ {{0}} ^ {{1}} n \ \ longrightarrow \ _ {{\ 90}} ^ {{233} } Th \ {\ xrightarrow [{22.3 \ min}] {\ beta ^ {-}}} \ _ {{\ 91}} ^ {{233}} Pa \ {\ xrightarrow [{26,967 \ d}] {\ beta ^ {-}}} \ _ {{\ 92}} ^ {{233}} U}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/e69ba8c959dd5ab6f587b18437ec429f67229b02)