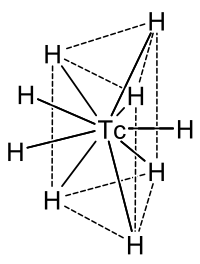Technetium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Technetium, Tc, 43 | |||||||||||||||||||||||||||||||||||||||||||||
| Element category | Transition metals | |||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 7 , 5 , d | |||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery gray metallic | |||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-26-8 | |||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-136-0 | |||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.305 | |||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 1.2 · 10 −15 ppm | |||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 98.9063 u | |||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 135 (185) pm | |||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 147 pm | |||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Kr ] 4 d 5 5 s 2 | |||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 7th.11938 (3) eV ≈ 686.92 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 15th.26 eV ≈ 1 472 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 29.55 eV ≈ 2 851 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 41.0 (1.7 eV) ≈ 3 956 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 57.0 (1.9) eV ≈ 5 500 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||||||||||||||||||||
| density | 11.5 g / cm 3 (25 ° C ) | |||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 3.9 10 −4 ) | |||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 2430 K (2157 ° C) | |||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 4538 K (4265 ° C) | |||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 8.63 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 550 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 23 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 4.54 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 51 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | −3 to 7 | |||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | 0.272 V (TcO 2 + 4 e - + 4 H + → Tc + 2 H 2 O) |
|||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.9 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | ||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||
Technetium is a chemical element with the element symbol Tc and atomic number 43. It occurs naturally on earth, albeit in very small quantities. Technetium was the first artificially produced element and therefore got its name derived from the ancient Greek word τεχνητός technētós ("artificial").
It is one of the transition metals , in the periodic table it is in the 5th period and the 7th subgroup (group 7) or manganese group . The discovery of the element by Walter Noddack , Ida Tacke and Otto Berg had already been reported in 1925 , who gave it the name Masurium . Technetium is therefore abbreviated to “Ma” in some older books.
All technetium isotopes are radioactive , which means that all atomic nuclei that contain 43 protons are unstable and decay. Technetium and the heavier promethium (61) are the only elements with a smaller atomic number than bismuth (83) that have this property.
history
For many years there was a gap between the elements molybdenum (42) and ruthenium (44) in the periodic table of the elements proposed by the Russian chemist Dmitri Mendeleev , which indicated a previously unidentified element. Mendeleev himself gave it the name Eka-Mangan and, with good approximation, predicted its mass, among other things. In the period that followed, numerous researchers tried to discover the missing element; its position in the periodic table reinforced the assumption that it was easier to find than other as yet undiscovered elements with higher atomic numbers.
Failed discoveries
The number of alleged evidence of the element, as well as the discoveries associated with the element, is unusually large. The first supposed discovery that was associated with technetium is that of Polinium in 1828 by Gottfried Osann . He believed that in addition to the actual discovery of ruthenium , he had also discovered an element that he called polinium. However, it soon turned out that the find was impure iridium . Due to its location in the periodic table , which was not yet fully known at the time , the discovery was associated with technetium.
The next putative element believed to be what would later become technetium was the ilmenium, discovered in 1846 . About this element , allegedly similar to niobium and tantalum (it was probably impure niobium), its discoverer R. Hermann, 30 years after its discovery and taking into account the periodic table, which has since been invented, was claimed to be the missing eka-manganese. The pelopium supposedly found by Heinrich Rose in 1847 was also mistaken for technetium.
The first false discovery that actually looked for the missing element with the atomic number 43 was the Davyum . In 1877, the Russian chemist reported Serge core discovering the missing element in platinum -Erz and gave the supposed element of the English chemist Sir Humphry Davy named Davyum . However, the find turned out to be a mixture of iridium , rhodium and iron .
Another supposed discovery was made in 1896 with Lucium , but it was yttrium . Finally, the Japanese chemist Masataka Ogawa concluded from an analysis of a mineral that there was nipponium (named after Nippon , the Japanese word for Japan ), which he believed to be the element with atomic number 43. Later analyzes indicated rhenium instead .
Erroneous evidence by Noddack, Tacke and Berg
The German chemists Walter Noddack , Ida Tacke and Otto Berg reported the discovery of the element 43 in 1925 and gave it the name Masurium , derived from Masuria , the home of Walter Noddack. At the Physikalisch-Technische Reichsanstalt Berlin, the group bombarded the mineral columbite with an electron beam and concluded from the X-ray spectra that element 43 was present. However, the signal observed was close to the detection limit and could not be reproduced by other working groups at the time. A preparative pure representation - in accordance with Mattauch's isobar rule - was not successful . The discovery was therefore not recognized. Even in 1933, several articles about the discovery of the elements used the name Masurium for element 43.
In the years between 1988 and 2005, the rejection of the discovery was repeatedly called into question. The physicist van Assche first revised the original Noddacks photo plate and came to the conclusion that in 1925 a proof of naturally occurring technetium from the spontaneous U-238 fission could indeed have been successful. John T. Armstrong from the US National Institute of Standards and Technology later simulated the experiments with a computer and came to results comparable to those of Noddack, Berg and Tacke. Support came from a work by David Curtis of Los Alamos National Laboratory , who demonstrated the very low natural occurrence of technetium using the methods of Noddack, Tacke, and Berg. The debate about the controversial first discovery therefore appeared to be open again for a short time. A little later, however, the most recent report on "rehabilitation" was withdrawn and the recent errors clearly stated. In the meantime it had become clear that the detection limit of the X-ray analytical method of the Noddacks was not sufficient to record naturally occurring traces of technetium.
Evidence from Segrè and Perrier
In 1937, 66 years after Dmitri Mendeleev had predicted many of the properties of technetium, the element was finally proven in an undisputed manner. Emilio Segrè and Carlo Perrier , both working at the University of Palermo , isolated the new element from a deuteron- bombarded molybdenum foil that Segrè had received at the beginning of the year from Ernest Lawrence of the University of California, Berkeley , USA:
- Molybdenum is converted into technetium with deuterons with neutron emission .
Segrè and Perrier named the first artificially produced element after the Greek word τεχνητός (transcription technētós ) for "artificial" as technetium and did not respond to requests from those responsible at the University of Palermo, who instead named the name after the Latin word for Palermo, Panormus Panormium had proposed.
Nuclear Medicine Applications
Powell Richards published the first study on the use of 99 m Tc (half-life 6 h) in nuclear medicine in June 1960 . 99 m Tc is obtained from the more stable 99 Mo (half-life 66 h) by means of technetium-99m generators . The first method for the economic separation of 99 Mo and 99 m Tc was developed in the 1960s by the American researchers Walter Tucker and Margaret Green at the Brookhaven National Laboratory .
Occurrence
Alien occurrence
In 1952, the American astronomer Paul Willard Merrill pointed spectroscopically in red giant stars of the S-; M and N classes after larger amounts of technetium. Because these stars are at the end of their evolution and are correspondingly old, but the longest half-life of a technetium isotope is only a little more than 4 million years, this was the first clear evidence that technetium and other heavy elements are produced by nuclear fusion inside stars arise. In main sequence stars like the Sun, however , the temperature inside the star is not high enough for the synthesis of elements heavier than iron . Conditions such as those found inside red giants are therefore essential for the synthesis of technetium.
Earthly occurrence
Ever since an element with the atomic number 43 was assumed to exist, natural occurrences have been searched for on earth. Only in 1961 was it possible to isolate about 1 ng of technetium from 5.3 kg of pitchblende from Katanga in Africa and to detect it spectrographically. The spontaneous fission of 238 U-nuclei creates element 43, whereby 1 ng of technetium is produced from 1 kg of pure uranium.
All technetium naturally present on earth is a temporary intermediate product of the nuclear decay of heavy atomic nuclei and decays itself again after a while. The occurrence of this element on earth is therefore not to be equated with that of a stable element. Overall, the technetium content of the earth's crust is only slightly higher than that of francium and astatine , both also radioactive elements that are only present on earth on a microgram scale.
In the biosphere , technetium occurs exclusively as a result of human activities. Above-ground nuclear weapons tests produced around 250 kg of technetium in the atmosphere up to 1994, plus around 1,600 kg that were released from reprocessing plants and nuclear reactors around the world up to 1986 . From 1995 to 1999, about 900 kg of the metal was discharged into the Irish Sea from the plant in Sellafield , UK , but since 2000 the legally permitted amount has been limited to 140 kg per year.
Technetium can only be detected in living things in exceptional cases, for example in lobsters from the heavily polluted Irish Sea. As a rule, it is only found in the human body in patients who have undergone a technetium-based nuclear medicine application.
Extraction and disposal
For medical purposes, technetium is usually obtained by neutron bombardment of 98 Mo:
The 99 Mo nuclei disintegrate into excited (metastable) 99 m Tc nuclei with the emission of beta radiation with a half-life of 2 days and 19 hours :
In practice, molybdenum is not an element, but rather in the form of its salt molybdate (MoO 4 2− ), which is adsorbed on aluminum oxide columns, the starting material for technetium production, so that it is not elemental technetium but the pertechnetate ion (TcO 4 - ) that is produced in typical concentrations of between 10 −6 and 10 −8 mol per liter. This is first separated from the remaining molybdate at its place of use before it can be reduced to the pure element by hydrogen gas H 2 in the presence of suitable ligands , organic substances that combine with technetium to form complexes . The complex-bound metastable isotope 99 m Tc with a half-life of only six hours changes to the ground state 99 Tc through emission of gamma radiation :
It is this radiation that is used in medical diagnostics.
In addition, several tons of technetium are produced every year in nuclear reactors from the fission of the uranium isotope 235 U; they make up about 6% of all fission products of a spent fuel element. The total amount of metal artificially produced up to the beginning of the 21st century is more than 78 tons and thus far above the natural technetium deposits.
Most of the metal produced by the reactor forms only undesirable radioactive waste . The isotope 99 Tc, which is quite long-lived with a half-life of more than 200,000 years and which is the dominant radiation source between around 10,000 and around 1,000,000 years after its generation, must be taken into account when it is stored. For disposal, geological formations that are considered stable, such as salt domes, are primarily considered; However, critics fear that the element could still be washed out by water into the environment. In addition, the possibility of transmutation , the conversion of the metal into other elements through neutron bombardment, is also considered.
For commercial use, technetium is obtained on a kilogram scale in reprocessing plants from spent nuclear fuel rods. To do this, it is first oxidized to pertechnetate TcO 4 - and then separated from uranium, plutonium and other compounds after a decay period of several years in dissolved form by extraction and ion exchange processes . The products ammonium pertechnetate NH 4 TcO 4 or ammonium technetium hexachloride (NH 4 ) 2 TcCl 6 can then be reduced to elemental technetium by thermal decomposition in hydrogen gas H 2 at high temperatures . Alternatively, the metal can be obtained by electrolysis of ammonium pertechnetate in sulfuric acid (H 2 SO 4 ) enriched with hydrogen peroxide (H 2 O 2 ) .
properties
Physical Properties
Technetium is a radioactive metal that appears dull gray in the common powder form. As a macroscopic solid, however, it has a silver-gray color and thus resembles the element platinum . Characteristic spectral lines of the technetium atoms are at 363, 403, 410, 426, 430 and 485 nanometers .
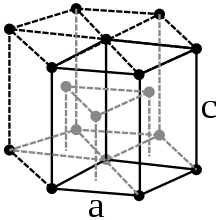
Both the melting point and the boiling point of 2157 and 4265 ° C are between the corresponding values of the group neighbors manganese and rhenium . Technetium crystallizes in the hexagonal crystal system (hexagonal close packing of spheres, magnesium type) in the space group P 6 3 / mmc (space group no.194) with the lattice parameters a = 275.3 pm and c = 440 pm as well as two formula units per unit cell .
Metallic technetium is slightly paramagnetic , i.e. its magnetic susceptibility Χ m is positive, the magnetic dipoles inside the material align parallel to an external magnetic field and the substance is drawn into it. At temperatures below 7.7 Kelvin, the pure element is a superconductor of the 2nd type , so it loses its electrical resistance ; However, even the smallest impurities raise this temperature to 11.2 Kelvin. The penetration depth of magnetic fields in the superconducting state is the second largest of all metals for technetium after niobium . Nuclear magnetic resonance examinations with technetium are possible due to the high sensitivity of the isotope 99 Tc.
Chemical properties
Technetium lies in its group in the periodic table between the two elements manganese and rhenium , but its chemical properties are only similar to the latter.
The technetium atom has seven valence electrons , one of them in the 5s orbital , the remaining six in the 4d orbital, the maximum oxidation state is therefore + VII. The first three ionization energies of 702, 1472 and 2850 kilojoules per mol (kJ / mol) are all below the corresponding values of the lighter group neighbor , manganese , which can be attributed qualitatively to the greater distance between the valence electrons and the nucleus and their reduced electrical interaction energy. In particular, the difference between the second and third ionization energy of 1378 kJ / mol is significantly lower than that of manganese of 1739 kJ / mol. Unlike this element, whose chemistry is essentially that of the doubly positively charged Mn 2+ ion, technetium is often found in other oxidation states. The most important are + IV, + V and + VII, in addition to which one can find compounds in which technetium has the oxidation number −I, 0, + I, + III or + VI, while the + II state so characteristic of manganese only rarely occurs .
In moist air, the metal slowly tarnishes due to oxidation . In addition to being flammable, the powder form is generally more reactive and combines violently with halogens . Technetium only dissolves in oxidizing acids such as concentrated sulfuric acid (H 2 SO 4 ) or nitric acid (HNO 3 ), but not in hydrochloric acid (HCl (aq) ) or hydrofluoric acid (HF (aq) ); The metal is stable in gaseous chlorine and hydrogen fluoride .
Isotopes
To date, 34 isotopes of technetium are known with mass numbers between 85 and 118. The longest-lived of these is 98 Tc with a half-life of 4.2 million years , followed by 97 Tc with a half-life of 2.6 million years and 99 Tc with a half-life of 211,100 years. The latter is also the most common and economically most important isotope and, with an activity of 620 million Becquerel per gram , releases a soft beta radiation of the energy 293.6 kiloelectron volts (keV).
The decay mechanism of isotopes with mass numbers below 98 is electron capture , so that molybdenum isotopes are formed; in the case of heavier technetium isotopes, however, beta decay and the formation of ruthenium isotopes occur. The only exception is 100 Tc, which can pass into another element via both decomposition pathways.
In addition to the isotopes, which are differentiated by their number of neutrons, there are a number of excited, metastable states such as 95 m Tc, 97 m Tc and 99 m Tc, which with half-lives of (in this order) 61 days, 90 days and 6.01 hours in the associated ground state pass over. The most important metastable isotope is 99 m Tc, which plays a major role in nuclear medicine .
The instability of technetium can be explained in terms of nuclear physics by the fact that its atomic number is odd and the neighboring elements molybdenum and ruthenium have very many stable isotopes ( Mattauch's isobar rule ).
use
Only small amounts of technetium are used economically; The largest part is used in medicine as a component of radiopharmaceuticals , but it is also used as a corrosion protection and beta radiation source.
Nuclear medicine
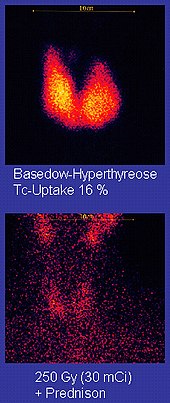
Due to its short half-life, the emitted gamma radiation with an energy of 140 keV and its ability to attach to many active biomolecules , metastable 99 m Tc is by far the most important nuclide used as a tracer for scintigraphic , i.e. image-creating, nuclear medicine examinations . For this purpose, organic ligands with a high tendency to bind to cells of the organ to be examined, or monoclonal antibodies , proteins of the immune system that attach themselves to selected antigens of tumor cells, are coupled to technetium and injected intravenously into the patient's bloodstream. The metal is concentrated in this way in the desired organs and tissues or the tumor to be examined; the characteristic gamma radiation may then with thallium doped sodium iodide - detectors registered and for non-invasive diagnosis, such as the labeled antibody by the tumor, are used. In this way, the brain , thyroid , lungs , liver , gallbladder , spleen , kidneys , bone tissue , but also parts of the intestine that are difficult to access can be examined. The coupling of technetium tin compounds to erythrocytes , the red blood cells, enables a diagnosis of diseases of the blood vessel system; Binding of technetium pyrophosphates to calcium deposits in the heart muscle tissue is used in the diagnosis of heart attack patients.
The high-energy gamma radiation emitted by 99 m Tc enables low dosage. After the examination, most of the technetium absorbed during a nuclear medicine diagnosis is excreted. The remaining 99 m Tc quickly breaks down into 99 Tc. This has a long half-life of 212,000 years and, because of the relatively soft beta radiation that is released when it decays, only contributes to a small additional radiation exposure over the remaining lifetime. In the United States -purpose diagnostic are about seven million individual doses per year for 99 m administered Tc.
Technetium for nuclear medicine purposes is usually obtained from technetium-99m generators due to its short 6-hour half-life . However, there are only five reactors in the world in which molybdenum-99 is obtained as the mother nuclide of technetium-99 (three in Europe, one in South Africa and one in Canada). Due to the great age of most of these reactors and the technical problems associated with them, several reactor failures have occurred recently, which has severely restricted the production of technetium. In the meantime (May 2010), nuclear medicine fears that these reactor problems could soon lead to a serious shortage of the isotope, which is important for tumor diagnosis.
Other uses
The non-excited isotope 99 Tc itself is used as an economically viable source for beta rays . It offers the advantage that no gamma radiation occurs when it decays, so that only relatively minor safety precautions are necessary.
In addition, technetium in the form of its salts is one of the best rust inhibitors: ammonium or potassium pertechnetate could be used as corrosion protection for steel . An addition of 55 ppm (millionths of a part) potassium pertechnetate (KTcO 4 ) in aerated deionized water protects this material from corrosion up to a temperature of 250 ° C. Because of the radioactivity of technetium, its potential application is limited to systems that are isolated from the environment, such as boiling water reactors .
Pertechnetates serve as important starting materials in technetium chemistry and also play a certain role as catalysts in inorganic chemistry.
Connections and reactions
→ Category: Technetium compound
In contrast to manganese, technetium hardly forms any cations . It is similar in this, as well as in its lower reactivity and in the ability to enter into covalent bonds, to its other group neighbor rhenium. In contrast to this, however, the high oxidation states are somewhat less resistant to reduction, the transition to a lower oxidation state through (formal) uptake of electrons.
Hydrido complex
When technetium reacts with hydrogen , the anionic , ie negatively charged hydrido complex [TcH 9 ] 2− is formed , the central technetium atom of which is located in a trigonal prism of hydrogen atoms, as shown opposite; there is also another hydrogen atom each perpendicular to the center of the three side faces. The charge can be balanced, for example, with two sodium (Na + ) or two potassium (K + ) ions .
Oxides
There are two different technetium oxides (TcO 2 and Tc 2 O 7 ). At temperatures of around 400–450 ° C, the metal reacts directly with oxygen to form pale yellow ditechnetium heptoxide:
The molecule consists of two technetium atoms connected to one another via an oxygen atom, which in turn are bound to the remaining oxygen atoms by three double bonds and is the anhydride of the pertechnetic acid HTcO 4 , which is formed when the oxide is dissolved in water.
The black technetium dioxide (TcO 2 ) can be produced by reducing ditechnetium heptoxide with elemental technetium or hydrogen.
Pertechnetic acid
Pertechnetic acid (HTcO 4 ) is formed when technetium heptoxide is dissolved in water or technetium in oxidizing acids such as nitric acid , concentrated sulfuric acid or aqua regia , a mixture of nitric acid and hydrochloric acid. The dark red, water-attracting (hygroscopic) substance is one of the strong acids and is strongly dissociated in water , so the proton is almost always transferred to a water molecule.
The remaining pertechnetate anion TcO 4 - consists of a technetium atom, which is located in the center of a tetrahedron , at the four corners of which are the oxygen atoms. In contrast to the permanganate ion MnO 4 - it is relatively stable to reduction, so that the colorless salts such as potassium (KTcO 4 ) or ammonium pertechnetate (NH 4 TcO 4 ) are only relatively weak oxidizing agents . Sodium , magnesium and calcium pertechnate are good, barium and ammonium pertechnate are moderate, while potassium and thallium pertechnate are only slightly soluble in water.
Pertechnetate can be reduced to technetate [TcO 4 ] 2− (purple) by using a reducing agent .
Halides and oxide halides
In addition to the technetium halides , in which technetium is bound to halogen atoms, numerous technetium oxide halides are known in which, in addition to the halogen atoms, oxygen is also bound.
The two fluorine compounds, the yellow technetium pentafluoride (TcF 5 ) and the technetium hexafluoride (TcF 6 ) of the same color, are formed through direct reaction of the starting materials . The two chlorine compounds, the green technetium hexachloride (TcCl 6 ) and the red technetium tetrachloride (TcCl 4 ), can also be synthesized directly . The latter is paramagnetic and is in polymerized form, i.e. as a chain of TcCl 4 subunits in a row , and can also be produced by reacting technetium heptoxide (Tc 2 O 7 ) with carbon tetrachloride (CCl 4 ). Important technetium halide salts are formed by the two anions [Tc 2 Cl 8 ] 2− and [TcCl 8 ] 3− . The most important bromine compound is the red-brown technetium tetrabromide TcBr 4 , and there is also the anion [Tc 2 Br 8 ] 2− .
The Technetiumoxidhalogenide are fluorine compounds Technetiumfluoridtrioxid TcO 3 F, Technetiumtrifluoriddioxid TcO 2 F 3 , Technetiumpentafluoridoxid TCOF 5 and Technetiumtetrafluoridoxid TCOF 4 , in which the metal in the oxidation states + VII and + VI occurs chlorine compounds Technetiumchloridtrioxid TcO 3 Cl, Technetiumtetrachloridoxid TcOCl 4 and technetium trichloride oxide TcOCl 3 with the oxidation states + VII, + VI and + V and for bromine and iodine the analogous compounds technetium bromide trioxide TcO 3 Br and technetium iodide trioxide TcO 3 I. In the latter substances, the central technetium atom has the maximum oxidation number + VII. Technetium trifluoride dioxide TcO 2 F 3 , like technetium trichloride oxide TcOCl 3 and technetium tribromide oxide TcOBr 3, is in polymerized form.
All halogen-oxygen compounds in technetium decompose easily on contact with water to pertechnetate and technetium dioxide. In particular, highly fluorinated compounds such as technetium pentafluoride oxide TcOF 5 can only be produced using strong fluorinating agents such as xenon hexafluoride XeF 6 or krypton difluoride KrF 2 , as the following reaction steps show by way of example:
- Ditechnetium heptoxide reacts with hydrogen fluoride to form technetium fluoride trioxide, oxonium ions and hydrogen difluoride (-1).
- Technetium fluoride trioxide reacts with xenon hexafluoride to form technetium trifluoride dioxide and xenon tetrafluoride oxide.
- Technetium trifluoride dioxide reacts with krypton difluoride to form technetium pentafluoride oxide, elemental krypton and oxygen.
Sulphides, selenides, tellurides
Technetium forms two different sulfides with sulfur . While technetium disulfide TcS 2 is produced by direct reaction of the starting materials, the black ditechnetium heptasulfide Tc 2 S 7 can be represented as follows:
- Pertechnetic acid reacts with hydrogen sulfide to form ditechnetium heptasulfide and water.
In this case, technetium is not reduced, unlike in the analogous reaction of manganese, in which the stable Mn 2+ ion is formed from MnO 4 - . Thermal decomposition of the heptasulphide leads to a breakdown into the disulphide and elemental sulfur:
With selenium and tellurium , technetium forms the analogous substances to technetium disulfide, i.e. technetium diselenide (TcSe 2 ) and technetium ditelluride (TcTe 2 ).
Cluster
There are two important technetium clusters , the Tc 6 and the Tc 8 cluster. In both of them two technetium atoms are linked by a triple bond. These pairs are arranged parallel to one another and bound to one another perpendicular to the alignment of the triple bond, so that the position of the single bonds results in two parallel equilateral triangles for the Tc 6 cluster and two parallel squares for the Tc 8 cluster. In the latter case, an additional single bond is aligned along a diagonal of these squares. Technetium atoms of both clusters all form six bonds; missing bonds can be satisfied by halogen atoms such as chlorine or bromine.
Complex compounds
Technetium is a component of numerous complex compounds that have been relatively well researched due to the element's importance for nuclear medicine.
One example is the technetium carbonyl complex Tc 2 (CO) 10 , which forms a white solid. It contains two technetium atoms that are weakly bonded to one another and are surrounded by five carbonyl ligands in octahedron symmetry , as shown opposite . The bond length of 303 pm is characteristically greater than the distance between two neighboring atoms in metallic technetium. Isostructural complexes, i.e. those with the same structure, are also found in the two neighboring elements manganese and rhenium. A technetium carbonyl complex in which technetium occurs in the negative oxidation state −I is [Tc (CO) 5 ] - , while the octahedral aquacomplex [Tc (H 2 O) 3 (CO) 3 ] + is formed in water .
An example of a complex with an organic ligand, which is used in practice in imaging processes in nuclear medicine, is given opposite and is characterized by a technetium atom located in the center of a carbon - nitrogen chain and connected via four nitrogen atoms, which is characterized by a double bond is bound to an oxygen atom. This technetium-oxygen unit can be replaced in the so-called nitrido complexes by a technetium-nitrogen unit in which there is a triple bond between a nitrogen and a technetium atom.
safety instructions
Classifications according to the CLP regulation are not available because they only cover the chemical hazard and play a subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
Precautions
According to the knowledge available to date, technetium has only a low chemical toxicity . However, as mentioned, all isotopes of the element are radioactive and must be stored in radiation protection containers according to their radiation intensity and marked as radioactive material. The beta radiation of the most common isotope, 99 Tc, is already stopped by glass; the radiation exposure caused by the soft X-rays released as bremsstrahlung is considered to be low if a safety distance of 30 centimeters is maintained. In contrast, inhaled technetium dust that settles in the lungs contributes to a higher risk of cancer. Laboratory work must therefore take place under a fume hood ; in addition, eye protection and the wearing of gloves are recommended.
literature
- Klaus Schwochau: Technetium: Chemistry and Radiopharmaceuticals. Wiley-VCH, Weinheim 2000, ISBN 3-527-29496-1 .
- CE Housecroft, AG Sharpe: Inorganic Chemistry. 2nd Edition. Pewson / Prentice Hall, 2005, ISBN 0-13-039913-2 , Chapter 22.8a, p. 666.
- RB King (Ed.): Encyclopedia of Inorganic Chemistry. Volume 8, Wiley, 1994, ISBN 0-471-93620-0 , p. 4094.
- Eric Scerri : A tale of seven elements , Oxford University Press, Oxford, 2013
Web links
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (Technetium) , unless otherwise stated .
- ↑ a b c d e Entry on technetium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on technetium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1339.
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-142-4-147. The values there are based on g / mol and are given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ a b c B. T. Kenna: The Search for Technetium in Nature. In: Journal of Chemical Education . 39 (2), 1962, pp. 436-442; doi: 10.1021 / ed039p436 .
- ^ A b Norman E. Holden: History of the Origin of the Chemical Elements and Their Discoverers. Brookhaven National Laboratory; Retrieved May 5, 2009.
- ^ Frederik AA de Jonge, Ernest KJ Pauwels: Technetium, the missing element. In: European Journal of Nuclear Medicine . 23 (3), 1996, pp. 336-344; doi: 10.1007 / BF00837634 .
- ↑ Serge core: LE NOUVEAU MÉTAL "LE DAVYUM". In: La Nature . No. 234, November 24, 1877, pp. 401-402.
- ↑ HK Yoshihara: Discovery of a new element 'nipponium': re-evaluation of pioneering works of Masataka Ogawa and his son Eijiro Ogawa. In: Spectrochimica Acta Part B: Atomic Spectroscopy . 59 (8), 2004, pp. 1305-1310; doi: 10.1016 / j.sab.2003.12.027 .
- ^ Hans Zettler: Masurium - a name that nobody mentions anymore. Why element 43 is called technetium. Rehabilitation by W. Noddack and I. Tacke. In: FAZ . February 22, 1989.
- ^ A b Peter van der Krogt: Elementymology and Elements Multidict, "Technetium". Retrieved May 5, 2009 .
- ^ A b John T. Armstrong: Technetium. In: Chemical & Engineering News. 2003.
- ↑ Kevin A. Nies: Ida Tacke and the warfare behind the discovery of fission. 2001, accessed May 5, 2009.
- ^ Mary Elvira Weeks: The Discovery of the Elements, XX: Recently Discovered Elements. In: Journal of Chemical Education . 10, 1933, pp. 161-170; doi: 10.1021 / ed010p161 .
- ^ Pieter HM Van Assche: The ignored discovery of the element Z = 43 ; Nuclear Physics A , 1988, 480 (2), pp. 205-214; doi: 10.1016 / 0375-9474 (88) 90393-4 .
- ^ Roberto Zingales: From Masurium to Trinacrium: The Troubled Story of Element 43. In: Journal of Chemical Education . 82, 2005, pp. 221-227; doi: 10.1021 / ed082p221 .
- ^ Fathi Habashi: The History of Element 43 — Technetium and reply by Roberto Zingales in: Journal of Chemical Education . 83, 2006, p. 213; doi: 10.1021 / ed083p213.1 .
- ^ PK Kuroda: A Note on the Discovery of Technetium ; Nuclear Physics A , 1989, 503 , pp. 178-182; doi: 10.1016 / 0375-9474 (89) 90260-1 .
- ^ Günter Herrmann: Technetium or masurium - a comment on the history of element 43 ; Nuclear Physics A , 1989, 505 , pp. 352-360; doi: 10.1016 / 0375-9474 (89) 90379-5 .
- ^ A b c John Emsley: Nature's Building Blocks: An AZ Guide to the Elements. Oxford University Press, New York 2001, ISBN 0-19-850340-7 , pp. 422-425.
- ^ C. Perrier, E. Segrè: Technetium: The Element of Atomic Number 43. In: Nature . 159, 1947, p. 24; doi: 10.1038 / 159024a0 .
- ^ S. Paul, W. Merrill: Spectroscopic Observations of Stars of Class S. In: The Astrophysical Journal . 116, 1952, pp. 21-26; doi: 10.1086 / 145589 .
- ↑ a b K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, pp. 7-9.
- ↑ a b c David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-35.
- ^ Charlotte E. Moore: Technetium in the Sun. In: Science . 114, No. 2951, 1951, pp. 59-61; doi: 10.1126 / science.114.2951.59 ; PMID 17782983 .
- ^ Paul Dixon, David B. Curtis, John Musgrave, Fred Roensch, Jeff Roach, Don Rokop: Analysis of Naturally Produced Technetium and Plutonium in Geologic Materials. In: Analytical Chemistry . 69 (9), 1997, pp. 1692-1699; doi: 10.1021 / ac961159q .
- ↑ D. Curtis: Nature's uncommon elements: plutonium and technetium. In: Geochimica et Cosmochimica Acta . 63 (2), 1999, pp. 275-285; doi: 10.1016 / S0016-7037 (98) 00282-8 .
- ^ A b c d K. Yoshihara: Technetium in the Environment. In: K. Yoshihara, T. Omori (Eds.): Technetium and Rhenium - Their Chemistry and Its Applications. (= Topics in Current Chemistry . Vol. 176). Springer-Verlag, Berlin / Heidelberg 1996, ISBN 3-540-59469-8 .
- ↑ Keiko Tagami: Technetium-99 Behavior in the Terrestrial Environment - Field Observations and Radiotracer Experiments. ( Memento of July 16, 2011 in the Internet Archive ) In: Journal of Nuclear and Radiochemical Sciences . 4, 2003, pp. A1-A8.
- ↑ John D. Harrison, Alan Phipps: Gut transfer and doses from environmental technetium. In: J. Radiol. Prot. 21, 2001, pp. 9-11; doi: 10.1088 / 0952-4746 / 21/1/004 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Atomic, Molecular, and Optical Physics, pp. 10-75.
- ↑ K. Schubert: A model for the crystal structures of the chemical elements. In: Acta Crystallographica B . 30, 1974, pp. 193-204; doi: 10.1107 / S0567740874002469 .
- ↑ a b c S. J. Rimshaw: The Encyclopedia of the Chemical Elements . Ed .: Cifford A. Hampel. Reinhold Book Corporation, New York 1968, pp. 689-693 .
- ^ SH Autler: Technetium as a Material for AC Superconductivity Applications. In: Proceedings of the 1968 Summer Study on Superconducting Devices and Accelerators. accessed on May 5, 2009 (PDF)
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, p. 96.
- ↑ a b Technetium, Nuclides / Isotopes. Retrieved May 5, 2009 .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Nuclear and Particle Physics, pp. 11-106.
- ↑ K. Schwochau: Technetium radiopharmaceuticals: Fundamentals, synthesis, structure and development. In: Angewandte Chemie International Edition . 33 (22), 1994, pp. 2258-2267; doi: 10.1002 / anie.199422581 .
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, p. 414.
- ^ A b Joseph F. Smith: Technetium heart scan. ( Memento of September 8, 2002 in the Internet Archive )
- ↑ Jonathan R. Dilworth, Suzanne J. Parrott: The biochemical chemistry of technetium and rhenium. In: Chemical Society Reviews . 27, 1998, pp. 43-55; doi: 10.1039 / a827043z .
- ↑ Bottlenecks in tumor medicine. Cancer doctors are running out of diagnostic tools. In: Der Spiegel. May 11, 2010.
- ^ Norbert Lossau: Cancer diagnosis: There is a threat of a shortage of technetium-99. In: welt.de . August 2, 2017, accessed October 7, 2018 .
- ↑ EPA: 402-b-04-001b-14-final. (PDF; 1.6 MB) Marlap, July 2004, accessed on August 4, 2008 .
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, p. 91.
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, pp. 127-136.
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, p. 104.
- ↑ B. Krebs: Technetium (VII) oxide: A transition metal oxide with a molecular structure in the solid state. In: Angewandte Chemie . 81 (9), 1969, pp. 328-329; doi: 10.1002 / anie.19690810905 .
- ^ AY Herrell, RH Busey, KH Gayer, K. Schwochau, S. Gutzeit: Technetium (VII) Oxide. In: Inorganic Syntheses . Vol. XVII, 1977, ISBN 0-07-044327-0 , pp. 155-158; doi: 10.1002 / 9780470132487.ch41 .
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, p. 108.
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1623.
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, pp. 112-113.
- ↑ KE German, SV Kryutchkov: Polynuclear technetium Halide cluster. In: Russian Journal of Inorganic Chemistry . 47 (4), 2002, pp. 578-583.
- ↑ JC Hileman, DK Huggins, HD Kaesz: technetium carbonyl. In: Journal of the American Chemical Society . 83 (13), 1961, pp. 2953-2954; doi: 10.1021 / ja01474a038 .
- ^ Marcia F. Bailey, Lawrence F. Dahl: The Crystal Structure of Ditechnetium Decacarbonyl. In: Inorganic Chemistry . 4 (8), 1965, pp. 1140-1145; doi: 10.1021 / ic50030a011 .
- ^ D. Wallach: Unit cell and space group of technetium carbonyl, Tc 2 (CO) 10 . In: Acta Crystallographica . 15, 1962, pp. 1058-1058; doi: 10.1107 / S0365110X62002789 .
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, pp. 286, 328.
- ↑ Silva Jurisson, EO Schlemper, DE Troutner, LR Canning, DP Nowotnik, RD Neirinckx: Synthesis, characterization, and x-ray structural determinations of technetium (V) -oxo-tetradentate amine oxime complexes. In: Inorganic Chemistry . 25 (4), 1986, pp. 543-549; doi: 10.1021 / ic00224a031 .
- ↑ K. Schwochau: Technetium: Chemistry and Radiopharmaceuticals. 2000, p. 40.





![\ mathrm {^ {99} _ {39} Y \ \ xrightarrow [1 {,} 47 \, s] {\ beta ^ {-}} \ ^ {99} _ {40} Zr \ \ xrightarrow [2 {, } 1 \, s] {\ beta ^ {-}} \ ^ {99} _ {41} Nb \ \ xrightarrow [15 {,} 0 \, s] {\ beta ^ {-}} \ ^ {99} _ {42} Mon \ \ xrightarrow [65 {,} 94 \, h] {\ beta ^ -} \ ^ {99} _ {43} Tc \ \ xrightarrow [211100 \, a] {\ beta ^ {-} } \ ^ {99} _ {44} Ru}](https://wikimedia.org/api/rest_v1/media/math/render/svg/70963d83d65b0763fa88e9b7f7880a3fbe0762c3)