biosphere

The biosphere [ bioˈsfɛːrə ] (from Greek βίος bíos 'life' and σφαίρα sphaira 'sphere') denotes the totality of all spaces of a heavenly body in which living beings occur. Usually the term refers to the earth's biosphere . The term has undergone two changes in meaning over the past century. At the moment it is understood primarily in an ecological meaning within the life sciences . As a result, biosphere is used synonymously with the terms biogeosphere , geobiosphere and ecosphere .
Except for the earth, the presence of living beings or at least traces of life has not been proven for any other planet, dwarf planet, asteroid or moon . According to the current state of knowledge, a biosphere seems to be a very rare phenomenon that makes the earth unique, at least in the local solar system.
The biosphere is thought of as a thin shell ( sphere ) that penetrates an outer area of the earth. It extends approximately from 5 km below the earth's surface to 60 km above the earth's surface, ie from the upper lithosphere to the lower edge of the mesosphere . Their outer areas in depth and in height are inhabited exclusively by microorganisms .
Because of its enormous size and complexity, aspects of the biosphere protrude into the research fields of many different natural sciences. The biosphere is a real research focus, at least for those natural sciences that move in the transition field between biology and geosciences - landscape ecology / geoecology and geobiology . In addition, there is biogeochemistry , which deals with the material cycles within the biosphere. And astrobiology tries to find out which conditions lead to the formation of a biosphere and how its presence on other celestial bodies could be recognized.
The two scientists Lynn Margulis and James Lovelock developed the Gaia hypothesis in the mid-1960s . It says that the terrestrial biosphere can be viewed as a holistic organism that creates and maintains the conditions that enable not only life, but also evolution . However, this theory is rejected by many scientists, although many of its predictions have already been verified.
Since the emergence of human civilizations , the anthropogenic influence on the natural spaces of the earth has increased. In 1976, the American biologist Raymond Dasmann coined the terms " ecosystem people and biosphere people " . The former are the ethnic groups living close to nature , which only influence one or a few ecosystems, while the latter are the modern societies which, due to global economic ties, have an impact on the most diverse habitats around the world. Since this influence on the biosphere has become an essential factor since 1800 at the latest, as the global environmental changes caused by humans show, there is discussion of proclaiming the beginning of a new geological age - called the Anthropocene . However, a large number of scientists view current trends for the biosphere with great skepticism and concern.
term

The terrestrial biosphere describes the space on planet earth in which life occurs. Life is dependent on interacting with its environment. To survive, living beings have to exchange substances and energy with their inanimate environment and with each other. They have to create so-called ecosystems . This is a fundamental property of living beings. Life would not be possible without ecosystem interactions. That is why life inevitably changes the furnishings of the room in which it is located. Since living beings have settled worldwide, the biosphere can be understood as the space of a global ecosystem.
- The terrestrial biosphere describes the space of the planet earth in which life occurs: The space together with the totality of terrestrial organisms and their inanimate environment and the interactions of living beings with one another and with their inanimate environment.
The existence of a global ecosystem was first recognized by the Russian geoscientist Vladimir Ivanovich Vernadsky . To name it, he used a word that had previously been invented by the Austrian geologist Eduard Suess : biosphere .
The biosphere can be divided into three major sub-units. The deep biosphere describes the ecosystems of the lithosphere below the earth's surface and soil. The hydrobiosphere describes the parts of the body of water that are populated and influenced by living things. The third sub-unit, the geobiosphere, describes the parts of the mainland that are populated and influenced by living things. Because the entire biosphere is sometimes named with the same word, the geobiosphere can be misunderstood.
Biosphere of microorganisms
The biosphere extends up to the lower edge of the mesosphere. The environmental conditions can vary greatly within the biosphere . Therefore, not all areas of the biosphere can be populated equally well by all living things. Multicellular organisms ( metabionta ) in particular can thrive permanently - and of course in the company of many microorganisms - only in regions in which there are relatively mild temperatures, pressures, radiation values, pH values and the like and in which there is sufficient supply of water and food.
In contrast, the environmental conditions in the outer biospheric zones are becoming increasingly extreme. Only microorganisms can exist there. In even harsher environmental conditions, even such resistant microbes can only exist in permanent stages . The permanent stages mark the outer limits of the biosphere.
- The terrestrial biosphere describes the space on planet earth in which microorganisms occur.
Biosphere of biomes
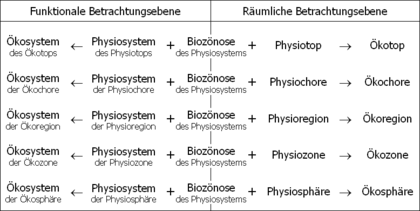
Living beings form biocenoses (communities) with one another. There are many mutual relationships among the members of a biocenosis, which are summarized as biotic eco-factors . Biocenoses inhabit physiotopes (material places) together. A physiotope is a small section of space with a homogeneous appearance, which is characterized by a certain, uniform physiosystem (location). With Physio system all of the salient in a landform is abiotic Ecofactors referred.
The members of the biocenosis interact with each other and with their physical system. They form a common network of effects. This structure of effects is called the ecosystem.
An ecosystem is a system , an association of interacting units. The system units of the ecosystem consist on the one hand of the living beings of the biocenosis and on the other hand of the inanimate things of the physical system. An ecosystem is an open system: substances and energy penetrate the ecosystem from outside, circulate for a certain time between the system units and finally leave it again.
A certain physiosystem only allows a certain biocenosis from those life forms that are adapted to it . However, the biocenosis gradually changes the expression of the abiotic eco-factors of the physical system. The biocenosis transforms the physiotope into an ecotope. The ecotope describes a real place in real space. It is the material equivalent to the term ecosystem, which itself is thought of as purely functional and abstract.
Ecotopes form common ecochores with similar neighboring ecotopes . Ecochores form common ecoregions with similar neighboring ecochores . Ecoregions form common ecozones with similar neighboring ecoregions . The WWF differentiates 825 terrestrial ecoregions worldwide , which are distributed over 14 main biomes. In addition there are 426 ecoregions of freshwater and 232 ecoregions of the seas.

Every living being is part of an ecoregion. This also applies even if the ecoregions for all aquatic and certainly not for the purely microbially populated areas of the biosphere have not yet been named. According to the classic definition, the biocenosis of an ecoregion forms its biome .
- The terrestrial biosphere describes the entirety of all biomes on planet earth.
| technical term | general synonyms *: obsolete |
purely terrestrial synonyms *: obsolete |
|---|---|---|
| Ecosphere | Biosphere, biogeosphere / geobiosphere | - |
| Eco zone | - | Zone biome, main biome, biome type, vegetation zone |
| Ecoregion | Eu biome | - |
| Ecochore | Ecotope structure | - |
| Ecotope | Biotope | Geoecotope , Tesela |
| Ecosystem | Holozön *, Zön * | Biogeozönose * / Biogeozönose *, geo-ecosystem |
| Biocenosis | Biosystem | - |
| Physiotope | - | Tile *, geotope , landscape cell *, parcel |
| Physiosystem | Location | Geosystem |
Similar terms
The term biosphere is understood differently by different scientific disciplines. In the biosciences, the ecological biosphere term, which was invented by Vladimir Ivanovich Wernadski, has now largely gained acceptance. However, he did not succeed in the geosciences . To this day, the majority of them use a biosphere term that goes back to the French Jesuit Pierre Teilhard de Chardin . Teilhard de Chardin understood the biosphere exclusively as the totality of terrestrial organisms. As a result, he coined a purely biotic biosphere term.
In addition to the ecological biosphere term, there are also a number of similar terms. Some are congruent with him in terms of content. They are called biogeosphere , geobiosphere and ecosphere . While the terms biogeosphere and geobiosphere are comparatively rare, the word ecosphere is used frequently. In fact, some authors consider the ecosphere to be more appropriate than the biosphere to denote the space of the global ecosystem.
In addition, there is another group of terms related to the ecological biosphere term. In terms of content, however, they are not completely congruent with him. Instead, they go beyond it by encompassing other parts of the earth. These are the terms Gaia , System Earth and Bioplanet Earth .

Green frame: biosphere (ecosphere)
Orange frame: system earth
Extension
The envelope-like biosphere begins about 60 km above the earth's surface and ends about 5 km below the earth's surface. It begins in the lower rim of the mesosphere, pervades the remaining layers of the earth's atmosphere below and the upper parts of the hydrosphere, penetrates the pedosphere and ends in the upper part of the lithosphere, after a few kilometers in the earth's crust . At least if attention is paid to microorganisms, the biosphere extends over the entire surface of the earth, the seas and the seabed.
Vertical extension
According to the current state of knowledge, the upper limit of the terrestrial biosphere is slightly above the stratopause , in the lowest mesosphere at an altitude of 60 km. Certain microorganisms still occur there in permanent stages. At these atmospheric altitudes, they defy the low temperatures, which range from around −50 ° C (lower stratosphere ) to around 0 ° C (lower mesosphere), as well as the almost complete lack of water and strong ultraviolet radiation . It is currently assumed that the microorganisms found do not go through their entire life cycle that far from the earth's surface. Instead, they should only be whirled up from the earth's surface in various ways and then remain for some time in the stratosphere and the lowest mesosphere.
Below the stratosphere is the troposphere , the densest and lowest layer of the earth's atmosphere. Here, thanks to the natural greenhouse effect , the air has higher air temperatures and is relatively low in radiation due to the stratospheric ozone layer above it . For these reasons, the habitats of terrestrial organisms are located in the troposphere, mostly just below the level of the nival due to the temperature .
Below the troposphere, on the one hand, the soils of the pedosphere and, on the other hand, the waters of the hydrosphere . The soils are inhabited by a variety of soil organisms . Their habitat is limited at the bottom by the supply of soil water and soil air , whereby microorganisms penetrate the deepest. Intact but frozen microorganisms can still be found deep in the permafrost . Life forms exist in the waters down to the bottom and again many meters into the muddy bottom. In fact, a larger proportion of the Earth's total biomass occurs in the form of archaea and bacteria in ocean sediments. The more conspicuous members of aquatic life, however, reside in the upper and light-flooded water layers of the Epipelagial . Beyond this, the species and individual densities can become very low. This is especially true for the deep sea. However, their cold darkness is interrupted by volcanic islands and atolls that rise above the surface of the water. Undersea, guyots and seamounts provide habitats for many organisms; some of these undersea mountains can rise up into the epipelagic. Seen worldwide, seamounts are very common and take up an area the size of Europe. Taken together, they likely form one of the major major biomes. Depending on the depth of the water, diverse communities can be found on volcanic islands, atolls, seamounts and guyots , which in this way interrupt the desert-like character of the deep sea.
Below the soils and muddy water beds, the rocks of the lithosphere join. Here, simple cave ecosystems consisting of microorganisms and some multicellular organisms have been found in caves. All other communities in the lithosphere consist exclusively of microorganisms. Some live in oil deposits, coal seams, gas hydrates , in deep aquifers or in fine pores directly in the bedrock. Furthermore, at least certain permanent microbial stages also occur in salt domes. It can be assumed that the biosphere in the lithosphere extends down to the depth from which the ambient temperature geothermally rises above 150 ° C. At this temperature, it should definitely get too hot even for hyperthermophilic microbes. As a rule of thumb, it is assumed that the ambient temperature increases by 3 ° C per 100 meters of depth. According to this, the biosphere should end at a depth of about 5 km in the lithosphere. However, there are strong regional deviations from this rule of thumb.
Microbial ecosystems can also be preserved in subglacial lakes that have been completely sealed off from the environment by the overlying glacial ice. Microorganisms are also found deep in the glacial ice itself. It remains unclear to what extent they only survive there or show active life processes. In 2018, researchers from Oregon State University's Deep Carbon Observatory team estimated that around 70 percent of the total number of bacteria and archaea on earth live in the earth's crust.
Horizontal extension
The living beings are not distributed evenly over the biosphere. On the one hand, there are biomes with large species and individual densities. These include, for example, the tropical rainforests and coral reefs. On the other hand, there are also areas with very sparse macroscopic and limited microscopic life. These include the cold and dry deserts on land and the seabeds of the lightless and cold deep sea ( Bathyal , Abyssal , Hadal ) in the seas . However, insular areas of higher biodiversity are interspersed within the desert areas : water oases in the dry deserts, post-volcanic phenomena ( thermal springs , solfataras , fumaroles , mofets ) in the cold deserts, as well as hydrothermal springs ( black smokers, white smokers ) and methane springs ( cold seas ) on the seabed the deep sea.
construction
Space with life is only a thin shell of the earth . Measured against the total earthly volume, the biosphere has only a tiny volume. Because terrestrial organisms have certain demands on their abiotic environment. Most areas of the world cannot meet the requirements.
The demands of living beings start with the space requirements. You can only stay in places that provide enough space for your body size. If there is enough space, the location must also offer suitable opportunities to be in the room. Which possibilities are suitable differs from life form to life form. Trees need enough root space and seaweed attachment points on the sea floor, while phytoplankton can get by with the free water body. The demands on the place of residence can change depending on the season and age.
Example: Adult king albatrosses need some space for their three meter wide wings. They roam the low layers of air above the open ocean. There they mainly prey on squid snails, drink sea water, sleep in flight or swim on the surface of the sea. Adult king albatrosses do not need a permanent settlement. However, this changes seasonally. Because they fly to the mainland every two years. There they court, occupy a breeding site, incubate an egg for 79 days and protect the still very defenseless young birds in the first five weeks of life. Then the parent birds fly out to sea again. However, they return to the hatchery at irregular intervals to feed the young birds. The young birds have to stay on land until they fledge after 236 days and follow their parents: The demands of the king albatrosses on their location in the biosphere change seasonally and with age.
Furthermore, the abiotic eco-factors (physical system, location) at the place of residence must move in ranges that are tolerable for earthly forms of life. This applies in an outstanding way to the supply of thermal energy and liquid water and subordinately to the other abiotic eco-factors. In addition, the place of residence must also ensure that living beings are fed. Autotrophic organisms must have sufficient building materials (nutrient salts) and heterotrophic organisms sufficient nutrients .
In the course of the earth's history, life forms have evolved very different body sizes, settlement methods, physical system requirements and diets. The same conditions do not prevail everywhere within the biosphere. Therefore, no living being occurs in all places in the biosphere. Lifestyles with similar or complementary adaptations can be found together at the same place of residence. Together they form ecoregions (EU biomes) and eco zones (zonobiomes).
The location of the mainland's eco-zones depends on the major climate. The major climate depends on the latitude (→ lighting zones ), on the distance to the sea (→ oceanity / continentality ) and possibly on high mountains that hold back precipitation (→ climate divide ). Overall, the eco-zones run roughly parallel to the width of a circle.
The location of the ocean's eco-zones ( realms ) depends on the near-surface water temperature. In addition, it must be taken into account that the coasts of the continents or the sheer expanse of the oceans represent barriers for many marine organisms that limit their expansion. A total of twelve marine eco-zones are distinguished worldwide. Within a marine ecozone there are, as it were, desert ecoregions next to ecoregions of great organismic abundance. The reason for this is that the same trophic conditions do not prevail everywhere in the oceans: Phytoplankton can only flourish extensively in the sea sections with a rich supply of building materials. Phytoplankton is at the base of the marine food web . As a result, the other marine life forms are particularly numerous there. Sections of the sea with high concentrations of building materials are upwelling areas in which deep water rich in building materials rises to the surface. Large amounts Walkot can produce a similar effect ( whale pump ).
Organismic structure
The size of the biosphere is primarily determined by microorganisms. Only permanent stages of microbes that are immune to inhospitable conditions are found at the outer limits of the biosphere. This applies to the mesosphere and stratosphere as well as to permafrost soils, salt domes and deep glacial ice. But many ecosystems that consist exclusively of microorganisms can also be found within the biospheric boundaries. This applies to all communities within the lithosphere, i.e. to deposits of crude oil, coal and gas hydrate as well as to deep aquifers, deeper sea sediment layers and ecosystems in the plain solid rock. The microorganisms also keep all rooms occupied that are also inhabited by multicellular animals. They even live on and in these metabionts, on the skin and rhizosphere as well as on leaves and in the digestive tract. The terrestrial biosphere turns out to be the sphere of microorganisms everywhere and especially in its more extreme areas. In comparison, the habitat of the metabionts appears to be very limited.
Trophic structure
Strictly speaking, the biosphere consists of many ecosystems that are more or less closely interlinked. In every ecosystem, living beings fulfill one of three different trophic functions : Primary producers - also known as autotrophs - build biomass from low-energy building materials . This biomass is then eaten by consumers . There is extensive inventory waste during production and consumption . The inventory waste is broken down by organisms of the third trophic function, the destructors , down to the low-energy building materials. The building materials can then be used again by the primary producers to build new biomass.
The existence of consumers and destructors depends on the existence of primary producers. Complete ecosystems can only develop in places where primary producers can find suitable living conditions . Ultimately, this applies to the entire biosphere. The extent and existence of the entire biosphere is spatiotemporally dependent on the presence of the primary producers.
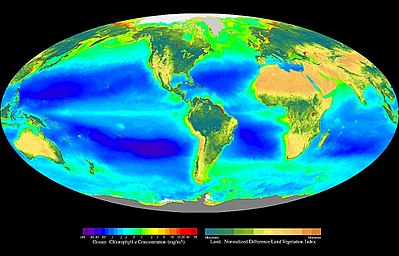
The most noticeable and important primary producers of the terrestrial biosphere are the photoautotrophic organisms. They perform photosynthesis in order to use light to produce their biomass from low-energy building materials. Land plants and algae (→ phototrophic organisms ) are among the best-known photoautotrophic organisms , with more than 99% of the total plant biomass being derived from land plants. The photoautotrophic primary production of the oceans is mainly done by non-calcifying haptophytes and cyanobacteria .
Photoautotrophic organisms are at the base of many terrestrial ecosystems. The biosphere shows its most species and individual-rich ecosystems in locations where plants or other photoautotrophic forms of life can exist. In the country in places where daylight can reach, but which are outside the cold deserts, outside the dry deserts and below the nival elevation. In the water in the euphotic zone of the epipelagial.
Beyond the areas with daylight, communities can only establish themselves in the long term if their phototrophic primary producers content themselves with the sparse glow from volcanic activities - or if they become completely independent of photoautotrophic biomass. Chemoautotrophic primary producers are then at the base of such completely light-independent ecosystems . Chemoautotrophic organisms also build their biomass from low-energy building materials. They don't get the energy they need from light, but from certain chemical reactions. The ecosystems that rely on chemoautotrophic primary producers include hydrothermal springs (Black Smokers, White Smokers), methane springs (Cold Seeps), subglacial lakes, caves that are completely isolated from the outside world and various microbial ecosystems deep in the bedrock (→ endoliths ).
The biosphere also includes spaces that are not directly part of the photoautotrophic or chemoautotrophic ecosystems. Instead, they lie between and outside of them. Because of the unfavorable living conditions, the rooms cannot be settled by primary producers. However, these inhospitable areas can temporarily be taken over by consumers who then return to autotrophically maintained ecosystems.
Example: On their annual migrations, many migratory birds pass through earths with extremely sparse autotrophic life. This is how white storks fly through the dry desert of the Sahara. Striped geese cross the vegetation-free main ridge of the Himalayas. Both bird species choose their winter and breeding areas again in habitats that are populated by plants. So they only stay temporarily outside photoautotrophically maintained ecosystems.
The vertical migration is similar to the annual bird migration : Depending on the time of day, many aquatic organisms migrate back and forth between the epipelagic and the underlying, low-light water layers. Some representatives of the phytoplankton migrate downwards at night in order to acquire building materials in the deeper water layers. At daybreak they return to the surface of the water. At the same time, zooplankton and some larger animals move in opposite directions . They swim towards the surface of the water under cover of darkness to hunt for prey and return to the depths at dawn to be safe from even larger predators.
In addition, waste is constantly draining from the autotrophically maintained ecosystems. The waste can still be recycled by destructors beyond the actual borders of those ecosystems. In this way, ecosystems can arise - and thus expand the biosphere - that are not based directly on primary producers present, but on waste that has flowed off. Typical examples of such ecosystems are the soils on which a wide variety of waste from terrestrial organisms is constantly falling. But also water beds and deeper water layers below the euphotic zone belong to it, to which stock waste trickles down from the epipelagial and from the banks. Particularly noteworthy here are the whale falls : Dead whales sink to the bottom of the sea and provide large amounts of usable waste for the deep-sea inhabitants. The whale carcasses also serve as intermediate stations for deep-sea organisms on their migrations between the chemoautotrophic ecosystems of the widely dispersed hydrothermal springs (smokers) and methane springs (cold seas). The degradation of waste in the sea takes place at lower rates even in the oxygen-poor zones ( oxygen minimum zones ) by appropriately adapted organisms. In addition to soils and water bottoms that are far from light, many caves also belong to the waste-based ecosystems, provided they are not completely sealed off from the outside world. Waste is brought into the caves in a variety of ways, a prominent example is bat guano .
See also
literature
- C. Beierkuhnlein: Biogeography . Stuttgart 2007, ISBN 978-3-8252-8341-4 .
- RJ Huggett: Ecosphere, biosphere or Gaia? What to call the global ecosystem. In: Global Ecology and Biogeography. 8, 1999, pp. 425-431. doi: 10.1046 / j.1365-2699.1999.00158.x [3] (PDF)
- B. Mason, CB Moor: Principles of Geochemistry . Stuttgart 1985, ISBN 3-8274-1262-5 .
- U. Kattmann: Bioplanet Earth. In: teaching biology. 299, 2004, pp. 4-13.
- V. Vernadsky: The Biosphere . Berlin / Heidelberg / New York 1998, ISBN 0-387-98268-X .
- H. Walter, S.-W. Breckle: Ecology of the Earth . Volume 1: Basics . Stuttgart 1991, ISBN 3-437-20454-8 .
- P. Ward: Gaia's evil sister. In: Spektrum der Wissenschaft 11, 2009, pp. 84–88. (online) .
Web links
Individual evidence
- ^ Raymond Dasmann: Toward a Biosphere Consciousness. In: Donald Worster (Ed.): The Ends of the Earth: Perspectives on Modern Environmental History. 2nd Edition. Cambridge University Press, New York 1989, ISBN 0-521-34365-8 , pp. 277-288, especially 277-279.
- ^ Sven Titz: Proclamation of the Anthropocene: A well-intentioned warning call. In: Neue Zürcher Zeitung . 4th November 2016.
- ↑ William J. Ripple, Christopher Wolf, Thomas M. Newsome, Mauro Galetti, Mohammed Alamgir, Eileen Crist, Mahmoud I. Mahmoud, William F. Laurance and 15,364 life scientists from 184 countries: World Scientists' Warning to Humanity: A Second Notice . In: BioScience . tape 67 , no. 12 , 2017, p. 1026-1028 , doi : 10.1093 / biosci / bix125 .
- ↑ a b H. Walter, S.-W. Breckle: Ecology of the Earth. Volume 1, Stuttgart 1991, p. 1.
- ↑ a b A. Kratochwil, A. Schwabe: Ökologie der Lebensgemeinschaften . Stuttgart 2001, ISBN 3-8252-8199-X , p. 102.
- ↑ В. И. Вернадский: биосфера [Biosfera]. Leningrad 1926.
- ↑ T. Gold: The deep, hot Biosphere. In: PNAS . 89, 1992, pp. 6045-6049 [1] (PDF)
- ↑ H. Walter, S.-W. Breckle: Ecology of the Earth. Volume 1, Stuttgart 1991, p. 2.
- ↑ HK Walter: The ecological systems of the continents (biogeosphere) . Stuttgart 1976, ISBN 3-437-30234-5 .
- ↑ S.-W. Breckle: Walter's vegetation of the earth: the ecological systems of the geo-biosphere . New York 2002, ISBN 3-540-43315-5 .
- ↑ a b H. Readers: Landscape Ecology. Stuttgart 1997, ISBN 3-8252-0521-5 , p. 222.
- ↑ a b Y. Yang, T. Itoh, S.-I. Yokobori, H. Shimada, S. Itahashi, K. Satoh, H. Ohba, I. Narumi, A. Yamagishi: Deinococcus aetherius sp. nov., isolated from the stratosphere. In: Int J Syst Evol Microbiol. 60, 2010, pp. 776-779. doi: 10.1099 / ijs.0.010876-0
- ↑ MJ Daly, EK Gaidamakova, VY Matrosova, JG Kiang, R. Fukumoto, D.-Y. Lee, NB Wehr, GA Viteri, BS Berlett, RL Levine, M. Otto: Small-Molecule Antioxidant Proteome-Shields in Deinococcus radiodurans. In: PLoS. 5 (2010), p. E12570. doi: 10.1371 / journal.pone.0012570
- ^ NA Campbell, JB Reece: Biology. Munich 2009, ISBN 978-3-8273-7287-1 , pp. 1544, 1547/1548.
- ↑ GA Zavarzin: Microbial Biosphere . doi : 10.1007 / 978-0-387-68656-1_2 In: NL Dobretsov, NA Kolchanov, A. Rozanov, GA Zavarzin (ed.): Biosphere Origin and Evolution . New York 2008, ISBN 978-0-387-68655-4 , pp. 25-42.
- ^ NA Campbell, JB Reece: Biology . Munich 2009, ISBN 978-3-8273-7287-1 , pp. 1611-1619.
- ↑ a b c H.-J. Klink: Vegetation Geography . Braunschweig 1998, ISBN 3-14-160282-4 , p. 103.
- ^ J. Schmithüsen: General Vegetation Geography. Berlin 1968, ISBN 3-11-006052-3 , p. 126.
- ↑ L. Finke: Landscape Ecology . Braunschweig 1994: 24, ISBN 3-14-160295-6 .
- ↑ C. Beierkuhnlein: Biogeography . Stuttgart 2009, ISBN 978-3-8252-8341-4 , p. 389.
- ↑ C. Beierkuhnlein: Biogeography . Stuttgart 2009, ISBN 978-3-8252-8341-4 , p. 211.
- ↑ a b A. Kratochwil, A. Schwabe: Ökologie der Lebensgemeinschaften. Stuttgart 2001, ISBN 3-8252-8199-X , p. 18.
- ↑ a b J. Schmithüsen: General vegetation geography . Berlin 1968, ISBN 3-11-006052-3 , p. 128.
- ↑ F. Müller: Ecosystem model concepts and ecosystem models in applied landscape ecology. In: R. Schneider-Sliwa, D. Schaub, G. Gerold (Eds.): Applied landscape ecology . Heidelberg 1999, ISBN 3-540-65938-2 , p. 30.
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 696.
- ↑ L. Finke: Landscape Ecology . Braunschweig 1994, ISBN 3-14-160295-6 , p. 24.
- ↑ a b H.-J. Klink: Vegetation Geography . Braunschweig 1998, ISBN 3-14-160282-4 , p. 105.
- ^ R. Böcker, H. Sukopp, H.-P. Blume, R. Grenzius, M. Horbert, A. Kirchgeorg, H.-J. Pachur, W. Ripl, A. v Stülpnagel: Ecological maps of Berlin - example Tegel and Tegeler See. In: B. Hofmeister, H.-J. Pachur, C. Pape, G. Reindke (Hrsg.): Berlin - contributions to the geography of a metropolitan area . Berlin 1986, p. 32.
- ↑ DM Olson, E. Dinerstein, E. Wikramanayake, N. Burgess, G. Powell, EC Underwood, J. d'Amico, I. Itoua, H. Strand, J. Morrison, C. Loucks, T. Allnutt, TH Ricketts, Y. Kura, W. Wettengel, K. Kassem: Terrestrial Ecoregions of the World: A New Map of Life on Earth. In: BioScience. 51, 2001, pp. 933-938 doi : 10.1641 / 0006-3568 (2001) 051 [0933: TEOTWA] 2.0.CO; 2
- Jump up ↑ R. Abell, ML Thieme, C. Revenga, M. Bryer, M. Kottelat, N. Bogutskaya, B. Coad, N. Mandrak, S. Contreras Balderas, W. Bussing, MLJ Stiassny, P. Skelton, GR Allen , P. Unmack, A. Naseka, R. Ng, N. Sindorf, J. Robertson, E. Armijo, JV Higgins, TJ Heibel, E. Wikramanayake, D. Olson, HL López, RE Reis, JG Lundberg, MH Sabaj Pérez, P. Petry: Freshwater Ecoregions of the World: A New Map of Biogeographic Units for Freshwater Biodiversity Conservation. In: BioScience. 58, 2008, pp. 403-414. doi: 10.1641 / B580507 (pdf)
- ↑ a b MD Spalding, HE Fox, GR Allen, N. Davidson, ZA Ferdana, M. Finlayson, BS Halpern, MA Jorge, A. Lombana, SA Lourie, KD Martin, E. McManus, J. Molnar, CA Recchia, J. Robertson: Marine Ecoregions of the World: A Bioregionalization of Coastal and Shelf Areas. In: BioScience. 57, 2007, pp. 573-583. doi: 10.1641 / B570707 [2] (PDF)
- ↑ A. Kratochwil, A. Schwabe: Ökologie der Lebensgemeinschaften . Stuttgart 2001, ISBN 3-8252-8199-X , p. 440.
- ↑ FE Clements: Chaper 2. In: FE Clements, VE Shelford: Bio-Ecology . New York 1939, p. 20.
- ↑ H. Walter, S.-W. Breckle: Ecology of the Earth. Volume 1, Stuttgart 1991, pp. 1-2.
- ↑ EP Odum: Ecology . Stuttgart 1999, ISBN 3-13-382303-5 , p. 3.
- ↑ DM Olson, E. Dinerstein, E. Wikramanayake, N. Burgess, G. Powell, EC Underwood, J. d'Amico, I. Itoua, H. Strand, J. Morrison, C. Loucks, T. Allnutt, TH Ricketts, Y. Kura, W. Wettengel, K. Kassem: Terrestrial Ecoregions of the World: A New Map of Life on Earth. In: BioScience. 51, 2001, p. 934 doi : 10.1641 / 0006-3568 (2001) 051 [0933: TEOTWA] 2.0.CO; 2
- ↑ EP Odum: Ecology. Stuttgart 1999, ISBN 3-13-382303-5 , p. 424.
- ^ A b I. Kronberg: Ecology of natural spaces. In: K. Munk (Ed.): Basic studies in biology. Biochemistry, cell biology, ecology, evolution. Heidelberg / Berlin 2000, ISBN 3-8274-0910-1 , pp. 17-1.
- ↑ W. Frey, R. Lösch: Textbook of Geobotany . Munich 2004, ISBN 3-8274-1193-9 , pp. 348/349.
- ↑ H. Walter, S.-W. Breckle: Ecology of the Earth. Volume 1, Stuttgart 1991, p. 27.
- ^ H. Readers: Landscape Ecology . Stuttgart 1997, ISBN 3-8252-0521-5 , p. 199.
- ↑ a b H. Readers: Landscape Ecology . Stuttgart 1997, ISBN 3-8252-0521-5 , p. 141.
- ↑ a b W. Frey, R. Lösch: Textbook of Geobotany . Munich 2004, ISBN 3-8274-1193-9 , p. 60.
- ↑ A. Kratochwil, A. Schwabe: Ökologie der Lebensgemeinschaften . Stuttgart 2001, ISBN 3-8252-8199-X , p. 90.
- ↑ H. Walter, S.-W. Breckle: Ecology of the Earth. Volume 1, Stuttgart 1991, p. 28.
- ^ H. Readers: Landscape Ecology . Stuttgart 1997, ISBN 3-8252-0521-5 , p. 163.
- ^ H. Readers: Landscape Ecology . Stuttgart 1997, ISBN 3-8252-0521-5 , p. 149.
- ↑ a b A. Kratochwil, A. Schwabe: Ökologie der Lebensgemeinschaften . Stuttgart 2001, ISBN 3-8252-8199-X , p. 94.
- ↑ a b H. Readers: Landscape Ecology . Stuttgart 1997, ISBN 3-8252-0521-5 , p. 148.
- ↑ RJ Huggett: Ecosphere, biosphere or Gaia? What to call the global ecosystem. In: Global Ecology and Biogeography. 8, 1999, pp. 425-431. doi: 10.1046 / j.1365-2699.1999.00158.x PDF
- ↑ JT Trevors, MH Saier Jr .: We Live and We Die. In: Water, Air, & Soil Pollution. 205, 2010, pp. 57/58. doi: 10.1007 / s11270-008-9662-7
- ↑ a b A. A. Imshenetsky, SV Lysenko, GA Kasakov, NV Ramkova: Resistance of stratospheric and mesospheric micro-organisms to extreme factors. In: Life Sci Space Res. 15, 1977, pp. 37-39. PMID 12596803 .
- ^ DJ Smith, DW Griffin, AC Schuerger: Stratospheric microbiology at 20 km over the Pacific Ocean. In: Aerobiologia. 26, 2010, pp. 35-46. doi: 10.1007 / s10453-009-9141-7
- ↑ S. Shivaji, P. Chaturvedi, Z. Begum, PK Pindi, R. Manorama, DA Padmanaban, YS Shouche, S. Pawar, P. Vaishampayan, CBS Dutt, GN Datta, RK Manchanda, UR Rao, PM Bhargava, JV Narlikar: Janibacter hoylei sp. nov., Bacillus isronensis sp. nov. and Bacillus aryabhattai sp. nov., isolated from cryotubes used for collecting air from the upper atmosphere. In: International Journal of Systematic and Evolutionary Microbiology. 59, 2009, pp. 2977-2986. doi: 10.1099 / ijs.0.002527-0
- ^ W. Lauer: Climatology . Braunschweig 1995, ISBN 3-14-160284-0 , p. 15.
- ^ W. Lauer: Climatology. Braunschweig 1995, ISBN 3-14-160284-0 , pp. 13, 66.
- ↑ M. Wainwright, S. Alharbi, NC Wickramasinghe: How do microorganisms reach the stratosphere? In: International Journal of Astrobiology. 5, 2006, pp. 13-15. doi: 10.1017 / S1473550406002825 (pdf)
- ^ W. Lauer: Climatology. Braunschweig 1995, ISBN 3-14-160284-0 , p. 27.
- ^ W. Lauer: Climatology. Braunschweig 1995, ISBN 3-14-160284-0 , p. 13.
- ^ F. Ehrendorfer: Geobotany. In: Strasburger textbook of botany. Stuttgart / Jena / Lübeck / Ulm 1998, ISBN 3-8274-0779-6 , p. 881.
- ↑ MT Madigan, JM Martinko: Brock microbiology . Munich 2006, ISBN 3-8273-7187-2 , p. 704.
- ↑ E. Vorobyova, V. Soina, M. Gorlenko, N. Minkovskaya, N. Zalinova, A. Mamukelashvili, D. Gilichinsky, E. Rivkina, T. Vishnivetskaya: The deep cold biosphere: facts and hypothesis. In: FEMS Microbiology Reviews. 20, 2006, pp. 277-290.
- ↑ a b DA Gilichinsky, GS Wilson, EI Friedmann, CP McKay, RS Sletten, EM Rivkina, TA Vishnivetskaya, LG Erokhina, NE Ivanushkina, GA Kochkina, VA Shcherbakova, VS Soina, EV Spirina, EA Vorobyova, DG Fyodorov-Davydov, B. Hallet, SM Ozerskaya, VA Sorokovikov, KS Laurinavichyus, AV Shatilovich, JP Chanton, VE Ostroumov, JM Tiedje: Microbial populations in Antarctic permafrost: biodiversity, state, age, and implication for astrobiology. In: Astrobiology. 7, 2007, pp. 275-311. doi: 10.1089 / ast.2006.0012
- ↑ a b J. F. Biddle, S. Fitz-Gibbon, SC Schuster, JE Brenchley, CH House: Metagenomic signatures of the Peru Margin subseafloor biosphere show a genetically distinct environment. In: PNAS. 105, 2008, pp. 10583-10588. doi: 10.1073 / pnas.0709942105
- ↑ JP Fischer, TG Ferdelman: Forty days in the water desert. In: Spectrum of Science. 03, 2010, pp. 16-18. (Items)
- ↑ CR McClain, L. Lundsten, M. Ream, JP Barry, A. DeVogelaere: Endemicity, biogeography, composition, and community structure on a Northeast Pacific Seamount. In: PLOS ONE. 4, 2009, p. E4141. doi: 10.1371 / journal.pone.0004141
- ↑ L. Lundsten, JP Barry, GM Cailliet, DA Clague, A. DeVogelaere, JB Geller: Benthic invertebrate communities on three seamounts off southern and central California. In: Marine Ecology Progress Series. 374, 2009, pp. 23-32. doi: 10.3354 / meps07745
- ^ P. Wessel, DT Sandwell, SS Kim: The Global Seamount Census. In: Oceanography. 23, 2010, pp. 24–33 (pdf)
- ↑ PJ Etnoyer, J. Wood, TC Shirley: How Large Is the Seamount biomes? In: Oceanography. 23, 2010, pp. 206-209 (pdf)
- ↑ FD Por: Ophel: a groundwater biome based on chemoautotrophic resources. The global significance of the Ayyalon cave finds, Israel. In: Hydrobiologia. 592, 2007, pp. 1-10. doi: 10.1007 / s10750-007-0795-2
- ↑ a b M. L. Porter, AS Engel, TC Kane, BK Kinkle: Productivity-Diversity Relationships from Chemolithoautotrophically Based Sulfidic Karst Systems. In: International Journal of Speleology. 38, 2009, pp. 27-40 (pdf)
- ↑ T. Kato, M. Haruki, T. Imanaka, M. Morikawa, S. Kanaya: Isolation and characterization of psychotrophic bacteria from oil-reservoir water and oil sands. In: Applied Microbiology and Biotechnology . 55, 2001, pp. 794-800. PMID 11525631 .
- ↑ E. Miranda-Tello, ML Fardeau, J. Sepúlveda, L. Fernandez, JL Cayol, P. Thomas, B. Ollivier: Garciella nitratireducens gen. Nov., Sp. nov., an anaerobic, thermophilic, nitrate- and thiosulfate-reducing bacterium isolated from an iolfield separator in the Gulf of Mexico. In: IJSEM. 53, 2003, pp. 1509-1514. doi: 10.1099 / ijs.0.02662-0
- ↑ M. Bonilla, ML Fardeau, JL Cayol, L. Casalot, B. Patel, P. Thomas, JL Garcia, B. Ollivier: Petrobacter succinatimandens gen. Nov., Sp. nov., a moderately thermophilic, nitrate-reducing bacterium isolated from an Australian oil well. In: IJSEM. 54, 2004, pp. 645-649. doi: 10.1099 / ijs.0.02732-0
- ^ A. Tabatabaee, Mazaheri Assadi M, AA Noohi, VA Sajadian: Isolation of Biosurfactant Producing Bacteria from Oil Reservoirs. In: Iranian J Env Health Sci Eng. 2, 2005, pp. 6-12. doi: 10.1002 / abio.370110405
- ↑ a b p Yuehui, Z. Béiwen, Z. Fan, W. Zhengliang, p Fuchang, Z. Lingge, X. Ting Sheng, Y. Longjiang: Analysis on the Microbial Diversity of Qinghai High Salt Content Oil reservoirs. In: ICBBE. (2008), pp. 780-783. doi: 10.1109 / ICBBE.2008.190
- ↑ a b JC Fry, B. Horsfield, R. Sykes, BA Cragg, C. Heywood, GT Kim, K. Mangelsdorf, DC Mildenhall, J. Rinna, A. Vieth, KG Zink, H. Sass, AJ Weightman, RJ Parkes: Prokaryotic populations and activities in an interbedded coal deposit, including a previously buried section (1.6 - 2.3 km) above ~ 150 Ma basement rock. In: Geomicrobiology Journal. 26, 2009, pp. 163-178. doi: 10.1080 / 01490450902724832
- ↑ a b B. A. Cragg, RJ Parkes, JC Fry, AJ Weightman, PA Rochelle ,. Maxwell JR: Bacterial populations and processes in sediments containing gas hydrates (ODP Leg 146: Cascadia Margin). In: Earth and Planetary Science Letters. 139, 1996, pp. 497-507. doi: 10.1016 / 0012-821X (95) 00246-9
- ↑ K. Haraa, T. Kakegawab, K. Yamashiroa, A. Maruyamac, J.-I. Ishibashid, K. Marumoe, T. Urabef, A. Yamagishia: Analysis of the archaeal sub-seafloor community at Suiyo Seamount on the Izu-Bonin Arc. In: Advances in Space Research. 35, 2005, pp. 1634-1642. doi: 10.1016 / j.asr.2005.04.111
- ^ A b G. J. Olson, WS Dockins, GA McFeters, WP Iverson: Sulfate-reducing and methanogenic bacteria from deep aquifers in montana. In: Geomicrobiology Journal. 2, 1981, pp. 327-340. doi: 10.1080 / 01490458109377772
- ^ A b D. Graur, T. Pupko: The Permian Bacterium that Isn't. In: Molecular Biology and Evolution. 18, 2001, pp. 1143–1146 (pdf)
- ↑ BA Schubert, TK Lowenstein, MN Timofeeff, MA Parker: How do prokaryotes survive in fluid inclusions in halite for 30 ky? In: Geology. 37, 2009, pp. 1059-1062. doi: 10.1130 / G30448A.1
- ↑ M. Kurr, R. Huber, H. Konig, HW Jannasch, H. Fricke, A. Trincone, JK Kristjansson, KO Stetter: Methanopyrus kandleri, gen. And sp. nov. represents a novel group of hyperthermophilic methanogens, growing at 110 ° C. In: Arch. Microbiol. 156, 1991, pp. 239-247. doi: 10.1007 / BF00262992
- ↑ K. Kashefi, DR Lovley: Extending the Upper Temperature Limit for Life. In: Science. 301, 2003, p. 934. doi: 10.1126 / science.1086823
- ↑ a b H. L. Ehrlich, DK Newman: Geomicrobiology . Boca Raton 2009, ISBN 978-0-8493-7906-2 , p. 50.
- ^ W. Zeil: Brinkmanns Abriß der Geologie, first volume: Allgemeine Geologie . Stuttgart 1980, ISBN 3-432-80594-2 , p. 217.
- ↑ JA Mikucki, A. Pearson, DT Johnston, AV Turchyn, J. Farquhar, DP Schrag, AD Anbar, JC Priscu, PA Lee: A Contemporary Microbially Maintained Subglacial Ferrous "Ocean". In: Science. 324, 2009, pp. 397-400. doi: 10.1126 / science.1167350
- ^ A b J. Loveland-Curtze, V. Miteva, J. Brenchley: Novel ultramicrobacterial isolates from a deep Greenland ice core represent a proposed new species, Chryseobacterium greenlandense sp. nov. In: Extremophiles. 14, 2010, pp. 61-69. doi: 10.1007 / s00792-009-0287-6
- ↑ Life Deep Underground Is Twice the Volume of the Oceans: Study. Retrieved April 2, 2019 .
- ↑ Life Thrives Within the Earth's Crust. Retrieved April 2, 2019 .
- ^ M. Bright, C. Arndt, H. Keckeisa, H. Felbeck: A temperature-tolerant interstitial worm with associated epibiotic bacteria from the shallow water fumaroles of Deception Island, Antarctica. In: Deep Sea Research Part II: Topical Studies in Oceanography. 50, 2003, pp. 1859-1871. doi: 10.1016 / S0967-0645 (03) 00095-X
- ↑ J. Imhoff, M. Hügler: Life at Deep Sea Hydrothermal Vents - Oases Under Water. In: The International Journal of Marine and Coastal Law. 24, 2009, pp. 201-208. doi: 10.1163 / 157180809X421789
- ↑ CM Santelli, BN Orcutt, E. Banning, W. Bach, CL Moyer, ML Sogin, H. Staudigel, KJ Edwards: Abundance and diversity of microbial life in ocean crust. In: Nature. 453, 2008, pp. 653-656. doi: 10.1038 / nature06899
- ↑ J. Sander: Species diversity of biofilms in Lost City. In: Naturwissenschaftliche Rundschau. 4, 2010, pp. 202/203.
- ↑ K. Knittel, T. Lösekann, A. Boetius, R. Kort, R. Amann: Diversity and Distribution of Methanotrophic Archaea at Cold Seeps. In: Applied and Environmental Microbiology. 71, 2005, pp. 467-479. doi: 10.1128 / AEM.71.1.467-479.2005
- ↑ LS Mullineaux, DK Adams, SW Mills, ST Beaulieu: Larvae from afar colonize deep-sea hydrothermal vents after a catastrophic eruption. In: PNAS. 107, 2010, pp. 7829-7834. doi: 10.1073 / pnas.0913187107
- ↑ J. Warham: Albatross Family. In: B. Grzimek (Ed.): Grzimeks Tierleben VII Vögel 1 . Zurich 1968, pp. 136, 139.
- ↑ C. Körner: Population and Vegetation Ecology. In: Strasburger textbook of botany. Heidelberg 2008, ISBN 978-3-8274-1455-7 , p. 1087.
- ↑ W. Frey, R. Lösch: Textbook of Geobotany . Munich 2004, ISBN 3-8274-1193-9 , pp. 267/268.
- ^ HU Sverdrup, MW Johnson, RH Fleming: The Oceans . New York 1942, pp. 628,786 (text)
- ^ J. Roman, JJ McCarthy: The Whale Pump: Marine Mammals Enhance Primary Productivity in a Coastal Basin. In: PLoS ONE. 5 (2010), p. E13255. doi: 10.1371 / journal.pone.0013255 (full text)
- ↑ G. Drews: Bacteria in the Antarctic dry valleys. In: Naturwissenschaftliche Rundschau. 3, 2010, pp. 143/144.
- ↑ DA Somerville, WC Noble: Microcolony Size of Microbes on Human Skin. In: J Med Microbiol. 6, 1973, pp. 323-328. doi: 10.1099 / 00222615-6-3-323
- ^ RN Harris, RM Brucker, JB Walke, MH Becker, CR Schwantes, DC Flaherty, BA Lam, DC Woodhams, CJ Briggs, VT Vredenburg, KPC Minbiole: Skin microbes on frogs prevent morbidity and mortality caused by a lethal skin fungus. In: ISME Journal. 3, 2009, pp. 818-824. doi: 10.1038 / ismej.2009.27
- ^ JAW Morgan, GD Bending, PJ White: Biological costs and benefits to plant – microbe interactions in the rhizosphere. In: Journal of Experimental Botany. 56, 2005, pp. 1729-1739. doi: 10.1093 / jxb / eri205
- ^ SE Lindow, MT Brandl: Microbiology of the Phyllosphere. In: Applied and Environmental Microbiology. 69, 2003, pp. 1875-1883. doi: 10.1128 / AEM.69.4.1875-1883.2003
- ↑ GL Simon, SL Gorbach: Intestinal flora in health and disease. In: Gastroenterology. 86, 1984, pp. 174-193. PMID 6357937 .
- ↑ F. Guarner: Gut flora in health and disease. In: The Lancet. 361, 2003, pp. 512-519. doi: 10.1016 / S0140-6736 (03) 12489-0
- ^ U. Sonnewald: Physiology. In: Strasburger textbook of botany. Heidelberg 2008, ISBN 978-3-8274-1455-7 , p. 274.
- ↑ H. Liua, I. Proberta, J. Uitzc, H. Claustred, S. Aris-Brosoue, M. Fradab, F. Nota, C. de Vargasa: Extreme diversity in noncalcifying haptophytes explains a major pigment paradox in open oceans. In: PNAS. 106, 2009, pp. 12803-12808. doi: 10.1073 / pnas.0905841106
- ↑ JT Beatty, J. Overmann, MT Lince, AK Manske, AS Lang, RE Blankenship, CL Van Dover, TA Martinson, FG Plumley: An obligately photosynthetic bacterial anaerobe from a deep-sea hydrothermal vent. In: PNAS. 102, 2005, pp. 9306-9310. doi: 10.1073 / pnas.0503674102 (pdf)
- ↑ FD Por: Ophel: a groundwater biome based on chemoautotrophic resources. The global significance of the Ayyalon cave finds, Israel. In: Hydrobiologia. 592, 2007, pp. 1-10. doi: 10.1007 / s10750-007-0795-2
- ^ WS Fyfe: The Biosphere Is Going Deep. In: Science. 273, 1996, pp. 448-450. doi: 10.1126 / science.273.5274.448
- ↑ B. Grzimek, E. Schüz: The white stork. In: B. Grzimek (Ed.): Grzimeks Tierleben VII Vögel 1 . Zurich 1968, p. 211.
- ↑ HG Klös, K. Klös: The other geese. In: B. Grzimek (Ed.): Grzimeks Tierleben VII Vögel 1 . Zurich 1968, p. 287.
- ^ JJ Cullen: Diel vertical migration by dinoflagellates: roles of carbohydrate metabolism and behavioral flexibility. In: Contr. Mar. Sci. 27, 1985, pp. 135–152 (pdf)
- ↑ EP Odum: Ecology . Stuttgart 1999, ISBN 3-13-382303-5 , p. 185.
- ^ W. Lampert: The adaptive significance of the vertical migration of zooplankton. In: Functional Ecology. 3, 1989, pp. 21-27. doi: 10.2307 / 2389671
- ^ MD Scheuerell, DE Schindler: Diel vertical migration by juvenile sockeye salmon: Empirical evidence for the antipredation window. In: Ecology. 84, 2003, pp. 1713-1720 doi : 10.1890 / 0012-9658 (2003) 084 [1713: DVMBJS] 2.0.CO; 2
- ↑ MM Yakimov, V. La Cono, R. Denaro, D'Auria G, F. Decembrini, KN Timmis, PN Golyshin, L. Giuliano: Primary producing prokaryotic communities of brine, interface and seawater above the halocline of deep anoxic lake L. 'Atalante, Eastern Mediterranean Sea. In: ISME J. 1, 2007, pp. 743-755. doi: 10.1038 / ismej.2007.83
- ^ CA Butman, JT Carlton, SR Palumbi: Whaling effects on deep-sea biodiversity. In: Conservation Biology. 9, 1995, pp. 462-464. doi: 10.1046 / j.1523-1739.1995.9020462.x
- ^ CR Smith, AR Baco: Ecology of whale falls at the deep-sea floor. In: Oceanography and Marine Biology Annual Review. 41, 2003, pp. 311–354 (pdf)
- ↑ CTS Little: Oases of the Deep Sea. In: Spectrum of Science. 03, 2011, pp. 74-79 (link)
- ^ AJ Gooday, BJ Bett, E. Escobar, B. Ingole, LA Levin, C. Neira, AV Raman, J. Sellanes: Habitat heterogeneity and its influence on benthic biodiversity in oxygen minimum zones. In: Marine Ecology. 31, 2010, pp. 125-147. doi: 10.1111 / j.1439-0485.2009.00348.x
- ↑ S. Negrea, V. Boitan: An ecological and biogeographycal overwiew of the terrestrial and aquatic subterranean environments from Romania. In: Travaux du Muskum National d'Histoire Naturelle. 43, 2001, pp. 396-397, 401.