Berkelium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Berkelium, Bk, 97 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Actinoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | Ac , 7 , f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-40-6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 247 u | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 170 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Rn ] 5 f 9 7 s 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.19785 (25) eV ≈ 598 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 11.9 (4) eV ≈ 1 150 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 21st.6 (4) eV ≈ 2 080 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 36.0 (4) eV ≈ 3 470 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 56.0 (1.9) eV ≈ 5 400kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | hexagonal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 14.78 g cm −3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1259 K (986 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 16.84 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +3 , +4 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −2.00 V (Bk 3+ + 3 e - → Bk) −1.08 V |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.30 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Berkelium is an artificially produced chemical element with the element symbol Bk and the atomic number 97. In the periodic table it is in the group of actinides ( 7th period , f-block ) and is also one of the transuranic elements . Berkelium was named after the city of Berkeley , California , where it was discovered. Berkelium is a radioactive metal with a silvery-white appearance. It was first created from the lighter element americium in December 1949 . It is produced in small quantities in nuclear reactors . It is mainly used to generate higher transuranic elements and transactinides .
history
Just as americium (atomic number 95) and curium (96) were discovered almost simultaneously in 1944 and 1945, the elements berkelium (97) and californium (98) were discovered in a similar way in 1949 and 1950 .
The experimenters Glenn T. Seaborg , Albert Ghiorso and Stanley G. Thompson produced the first cores in the 60-inch cyclotron at the University of California at Berkeley on December 19, 1949 . It was the fifth transurane to be discovered. The discovery was published at the same time as that of the Californium.
The choice of name obviously followed a common origin: Berkelium was named after the place where it was found, the city of Berkeley in California . The naming thus follows as with many actinides and the lanthanides : Terbium , which is exactly above Berkelium in the periodic table , was named after the Swedish city of Ytterby , where it was first discovered: It is suggested that element 97 be given the name berkelium (symbol Bk) after the city of Berkeley in a manner similar to that used in naming its chemical homologue terbium (atomic number 65) whose name was derived from the town of Ytterby, Sweden, where the rare earth minerals were first found. The name Californium was chosen for Element 98 in honor of the University and the State of California.
The most difficult steps in the preparation for the production of the element turned out to be the development of appropriate chemical separation methods and the production of sufficient quantities of americium for the target material.
The sample preparation was first carried out by applying americium nitrate solution (with the isotope 241 Am) to a platinum foil; the solution was evaporated and the residue then calcined to the oxide (AmO 2 ).
This sample was then bombarded with accelerated α-particles with an energy of 35 MeV for about 6 hours in a 60-inch cyclotron . A so-called (α, 2n) reaction produces 243 Bk and two free neutrons :
After bombardment in the cyclotron, the coating was dissolved and heated using nitric acid , then again precipitated as hydroxide with a concentrated aqueous ammonia solution and centrifuged off; the residue was again dissolved in nitric acid.
In order to achieve extensive separation of the americium, a mixture of ammonium peroxodisulphate and ammonium sulphate was added to this solution and heated to bring the dissolved americium to the +6 oxidation state . Unoxidized remaining americium was precipitated as americium (III) fluoride by adding hydrofluoric acid . In this way, accompanying curium is also precipitated as curium (III) fluoride and the expected element 97 (berkelium) as berkelium (III) fluoride . This residue was converted to the hydroxide by treatment with potassium hydroxide solution , which was then dissolved in perchloric acid after centrifugation .
The further separation took place in the presence of a citric acid / ammonium citrate buffer in a weakly acidic medium ( pH ≈ 3.5) with ion exchangers at an elevated temperature.
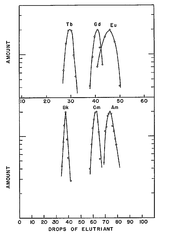
The chromatographic separation could only succeed on the basis of previous comparisons with the chemical behavior of the corresponding lanthanoids . In the event of a separation, the terbium emerges from a column before gadolinium and europium . If the chemical behavior of the Berkelium is similar to that of an Eka-Terbium , the element 97 in question should therefore appear first in this analogous position, correspondingly before Curium and Americium.
The further course of the experiment initially brought no result, as one was looking for an α-particle as the decay signature. Only the search for characteristic X-rays and conversion electrons as a result of electron capture brought the desired success. The result of the nuclear reaction was given as 243 Bk, although 244 Bk was initially thought to be possible.
In 1958, Burris B. Cunningham and Stanley G. Thompson isolated for the first time weighable quantities that were generated by long-term neutron irradiation of 239 Pu in the test reactor at the National Reactor Testing Station in Idaho .
Isotopes
Berkelium only has radionuclides and no stable isotopes . A total of 12 isotopes and 5 core isomers of the element are known. The longest lived are 247 Bk ( half-life 1380 years), 248 Bk (9 years) and 249 Bk (330 days). The half-lives of the remaining isotopes range from milliseconds to hours or days.
If one takes out, for example, the decay of the longest-lived isotope 247 Bk, then the long-lived 243 Am is initially formed by α-decay , which in turn changes to 239 Np through renewed α-decay . The further decay then leads via 239 Pu to 235 U, the beginning of the uranium-actinium series (4 n + 3).
- The times given are half-lives.
Occurrence
Berkelium isotopes do not occur naturally on Earth because their half-life is too short compared to the age of the Earth.
In addition to the first discovery of Einsteinium and Fermium in the remains of the first American hydrogen bomb, Ivy Mike , on November 1, 1952 on the Eniwetok Atoll , in addition to plutonium and americium, isotopes of curium, berkelium and californium were found, including the 249 Bk, the converts to 249 Cf due to the β decay . For reasons of military secrecy, the results were not published until later in 1956.
In nuclear reactors, the Berkelium isotope 249 Bk is mainly formed ; it decays almost completely to the Californium isotope 249 Cf with a half-life of 351 years during interim storage (before final disposal) . This counts as transurane waste and is therefore undesirable in final disposal.
Extraction and presentation
Berkelium is created by bombarding lighter actinides with neutrons in a nuclear reactor . The main source is the 85 MW High Flux Isotope Reactor (HFIR) at the Oak Ridge National Laboratory in Tennessee, USA, which is set up for the production of transcurium elements (Z> 96).
Extraction of Berkelium isotopes
Berkelium is formed in nuclear reactors from uranium ( 238 U) or plutonium ( 239 Pu) through numerous successive neutron captures and β-decays - to the exclusion of fission or α-decays.
An important step here is the (n, γ) - or neutron capture reaction, in which the generated excited daughter nuclide changes to the ground state by emitting a γ quantum . The free neutrons required for this are created by fission of other nuclei in the reactor. In this nuclear chemical process, the 239 Pu is first formed by an (n, γ) reaction followed by two β - decays . In breeder reactors this process is used to incubate new fissile material.
- The times given are half-lives.
For this purpose, the latter is irradiated with a neutron source that has a high neutron flux. The neutron fluxes that are possible here are many times higher than in a nuclear reactor. From 239 Pu, four successive (n, γ) reactions form 243 Pu, which decomposes to 243 Am through β-decay with a half-life of 4.96 hours . The 244 Am formed by a further (n, γ) -reaction finally decays to 244 Cm again by β-decay with a half-life of 10.1 hours . The next heavier isotopes are formed from 244 cm through further (n, γ) reactions in the reactor in smaller and smaller quantities.
However, the formation of 250 Cm in this way is very unlikely, since 249 Cm has only a short half-life and so further neutron captures are unlikely in the short time.
249 Bk is the only isotope of the Berkelium that can be formed in this way. It is formed by β-decay from 249 cm - the first curium isotope to undergo a β-decay (half-life 64.15 min).
By neutron capture, 250 Bk is formed from 249 Bk , but this already decays with a half-life of 3.212 hours through β-decay to 250 Cf.
The longest-lived isotope, the 247 Bk, cannot be produced in nuclear reactors, so that one often has to be content with the more accessible 249 Bk. Berkelium is only available in very small quantities worldwide today, which is why it has a very high price. This is about 185 US dollars per microgram 249 Bk.
The isotope 248 Bk was produced in 1956 from a mixture of curium nuclides by bombardment with 25 MeV α particles. Its existence with its half-life of 23 ± 5 hours was determined by the β-decay product 248 Cf.
247 Bk was made in 1965 from 244 cm by bombarding it with α-particles. A possible isotope 248 Bk could not be detected.
The Berkelium isotope 242 Bk was created in 1979 by bombarding 235 U with 11 B, 238 U with 10 B, and 232 Th with 14 N and 15 N, respectively . It converts to 242 cm by electron capture with a half-life of 7.0 ± 1.3 minutes . A search for an initially suspected isotope 241 Bk was unsuccessful.
Representation of elementary Berkelium
The first samples of berkelium metal were produced in 1969 by reducing BkF 3 at 1000 ° C with lithium in tantalum reaction apparatus .
Elemental berkelium can also be produced from BkF 4 with lithium or by reducing BkO 2 with lanthanum or thorium .
properties
In the periodic table , the Berkelium with atomic number 97 is in the series of actinides, its predecessor is the Curium , the following element is the Californium . Its analogue in the series of lanthanoids is terbium .
Physical Properties
Berkelium is a radioactive metal with a silvery-white appearance and a melting point of 986 ° C.
The modification α-Bk occurring under standard conditions crystallizes in the hexagonal crystal system in the space group P 6 3 / mmc (space group no. 194) with the lattice parameters a = 341.6 ± 0.3 pm and c = 1106.9 ± 0.7 pm and four formula units per unit cell , a metal radius of 170 nm and a density of 14.78 g / cm 3 . The crystal structure consists of a double hexagonal close packing of spheres (i.e. hcp) with the layer sequence ABAC and is therefore isotypic to the structure of α-La .
At higher temperatures, α-Bk changes to β-Bk. The β-modification crystallizes in the cubic crystal system in the space group Fm 3 m (No. 225) with the lattice parameter a = 499.7 ± 0.4 pm, a metal radius of 177 nm and a density of 13.25 g / cm 3 . The crystal structure consists of a cubic closest packing of spheres with the stacking sequence ABC, which corresponds to a face-centered cubic lattice (fcc).
The enthalpy of solution of Berkelium metal in hydrochloric acid under standard conditions is −600.2 ± 5.1 kJ mol −1 . Based on this value, the standard enthalpy of formation (Δ f H 0 ) was calculated for the first time from Bk 3+ (aq) to −601 ± 5 kJ mol −1 and the standard potential Bk 3+ / Bk 0 to −2.01 ± 0, 03 V.
Between 70 K and room temperature, Berkelium behaves like a Curie-Weiss paramagnet with an effective magnetic moment of 9.69 Bohr magnetons (µ B ) and a Curie temperature of 101 K. When cooling to about 34 K, Berkelium experiences a transition to an anti-ferromagnetic state. This magnetic moment nearly corresponds to the theoretical value of 9.72 μ B .
Chemical properties
Like all actinides, Berkelium is very reactive. However, it does not react quickly with oxygen at room temperature, which may be due to the formation of a protective oxide layer. However, it reacts with molten metals, hydrogen, halogens, chalcogens, and pentelides to form various binary compounds.
The trivalent oxidation state is most stable in aqueous solution , but tetravalent and divalent compounds are also known. Aqueous solutions with Bk 3+ ions have a yellow-green color, with Bk 4+ ions they are beige in hydrochloric acid solution and orange-yellow in sulfuric acid solution. A similar behavior can be observed for its lanthanide analogue terbium .
Bk 3+ ions show two sharp fluorescence peaks at 652 nm (red light) and 742 nm (dark red - near infrared) through internal transitions in the f-electron shell.
Cleavage
Unlike the neighboring elements curium and californium, berkelium is theoretically very poorly suited as a nuclear fuel in a reactor. In addition to the very low availability and the associated high price, an additional complication is that the cheaper isotopes with an even mass number only have a short half-life. The only possible even-numbered isotope, 248 Bk in the ground state, is very difficult to generate, and there is insufficient data on its cross- sections.
In principle, 249 Bk is able to maintain a chain reaction and is therefore suitable for a rapid reactor or an atomic bomb. The short half-life of 330 days together with the complicated extraction and high demand thwart corresponding attempts. The unreflected critical mass is 192 kg, with the water reflector still 179 kg, a multiple of the annual global production.
247 Bk can sustain a chain reaction in both a thermal and a fast reactor and, at 1380 years, has a sufficiently long half-life to serve as both nuclear fuel and fissile material for an atomic bomb . However, it cannot be hatched in a reactor and is therefore even more complex and costly to produce than the other isotopes mentioned. This is accompanied by an even lower availability, which, given the required mass of at least 35.2 kg (critical mass with steel reflector), can be seen as an exclusion criterion.
use

The use for Berkelium isotopes is mainly in basic scientific research. 249 Bk is a common nuclide for the synthesis of even heavier transuranic elements and transactinoids such as lawrencium , rutherfordium and boron . It also serves as a source for the isotope 249 Cf, which enables studies on the chemistry of California. It has preference over the more radioactive 252 Cf, which is otherwise generated by neutron bombardment in the high-flux isotope reactor (HFIR).
A 22 milligram sample of 249 Bk was made in a 250-day irradiation in 2009 and then cleaned in a 90-day process in Oak Ridge. This sample resulted in the first 6 atoms of the element Tenness at the United Institute for Nuclear Research (JINR), Dubna , Russia, after exposure to calcium ions in the U400 cyclotron for 150 days. This synthesis was a culmination of the Russian-American collaboration between JINR and Lawrence Livermore National Laboratory on the synthesis of elements 113-118, which began in 1989.
links
→ Category: Berkelium compound
Although the isotope 247 Bk has the longest half-life, the isotope 249 Bk is more easily accessible and is mainly used to determine the chemical properties.
Oxides
Berkelium has oxides of oxidation states +3 (Bk 2 O 3 ) and +4 (BkO 2 ).
Berkelium (IV) oxide (BkO 2 ) is a brown solid and crystallizes in the cubic crystal system in the fluorite structure in the space group Fm 3 m (space group no. 225) with the coordination numbers Cf [8], O [4]. The lattice parameter is 533.4 ± 0.5 pm .
Berkelium (III) oxide (Bk 2 O 3 ) is produced from BkO 2 by reduction with hydrogen:
It is a yellow-green solid with a melting point of 1920 ° C. It forms a body-centered cubic crystal lattice with a = 1088.0 ± 0.5 pm.
Halides
Halides are known to have the +3 and +4 oxidation states. The most stable level +3 is known for all compounds from fluorine to iodine and is also stable in aqueous solution. The tetravalent level can only be stabilized in the solid phase.
| Oxidation number | F. | Cl | Br | I. |
| +4 |
Berkelium (IV) fluoride BkF 4 yellow-green |
|||
| +3 |
Berkelium (III) fluoride BkF 3 yellow-green |
Berkelium (III) chloride BkCl 3 green |
Berkelium (III) bromide BkBr 3 yellow-green |
Berkelium (III) iodide BkI 3 yellow |
Berkelium (IV) fluoride (BkF 4 ) is a yellow-green ion compound and crystallizes in the monoclinic crystal system and is isotype with uranium (IV) fluoride .
Berkelium (III) fluoride (BkF 3 ) is a yellow-green solid and has two crystalline structures that are temperature-dependent (transformation temperature: 350 to 600 ° C). The orthorhombic structure ( YF 3 type ) can be found at low temperatures . At higher temperatures it forms a trigonal system ( LaF 3 type ).
Berkelium (III) chloride (BkCl 3 ) is a green solid with a melting point of 603 ° C and crystallizes in the hexagonal crystal system . Its crystal structure is isotype with uranium (III) chloride (UCl 3 ). The hexahydrate (BkCl 3 · 6 H 2 O) has a monoclinic crystal structure.
Berkelium (III) bromide (BkBr 3 ) is a yellow-green solid and crystallized at low temperatures in pUBR 3 type , at higher temperatures in the AlCl 3 -type .
Berkelium (III) iodide (BkI 3 ) is a yellow solid and crystallizes in the hexagonal system ( BiI 3 type ).
The oxyhalides BkOCl, BkOBr and BkOI have a tetragonal structure of the PbFCl type.
Chalcogenides and pentelides
Berkelium (III) sulfide (Bk 2 S 3 ) was prepared either by treating berkelium (III) oxide with a mixture of hydrogen sulfide and carbon disulfide at 1130 ° C, or by reacting metallic berkelium directly with sulfur. This produced brownish-black crystals with cubic symmetry and a lattice constant of a = 844 pm.
The pentelids of the Berkelium ( 249 Bk) of the BkX type have been shown for the elements nitrogen , phosphorus , arsenic and antimony . They are produced by the reaction of either berkelium (III) hydride (BkH 3 ) or metallic berkelium with these elements at elevated temperature in a high vacuum in quartz ampoules. They crystallize in the NaCl lattice with lattice constants 495.1 pm for BkN, 566.9 pm for BkP, 582.9 pm for BkAs and 619.1 pm for BkSb.
Other inorganic compounds
Berkelium (III) and Berkelium (IV) hydroxide are both stable as a suspension in 1 M sodium hydroxide solution and were examined spectroscopically. Berkelium (III) phosphate (BkPO 4 ) was presented as a solid which shows strong fluorescence when excited by an argon laser (514.5 nm line).
Other salts of Berkelium are known, e.g. B. Bk 2 O 2 S, (BKNO 3 ) 3 · 4 H 2 O, BkCl 3 · 6 H 2 O, Bk 2 (SO 4 ) 3 · 12 H 2 O and Bk 2 (C 2 O 4 ) 3 · 4 H 2 O. Thermal decomposition in an argon atmosphere at approx. 600 ° C (to avoid oxidation to BkO 2 ) of Bk 2 (SO 4 ) 3 12 H 2 O leads to body-centered orthorhombic crystals of Berkelium (III) - oxysulfate (Bk 2 O 2 SO 4 ). This connection is thermally stable up to at least 1000 ° C under protective gas.
Berkelium hydrides are made by reacting the metal with hydrogen gas at temperatures above 250 ° C. They form non-stoichiometric compositions with the nominal formula BkH 2 + x (0 <x <1). While the trihydrides have hexagonal symmetry, the dihydride crystallizes in an fcc structure with the lattice constant a = 523 pm.
Organometallic compounds
Berkelium forms a trigonal (η 5 –C 5 H 5 ) 3 Bk complex with three cyclopentadienyl rings , which can be synthesized by reacting berkelium (III) chloride with molten Be (C 5 H 5 ) 2 at about 70 ° C. It has a yellow color and orthorhombic symmetry with the lattice constants a = 1411 pm, b = 1755 pm and c = 963 pm and a calculated density of 2.47 g / cm 3 . The complex is stable up to at least 250 ° C and sublimates at approx. 350 ° C. However, the high level of radioactivity causes rapid destruction of the connections within a few weeks. A C 5 H 5 ring in the (η 5 –C 5 H 5 ) 3 Bk can be replaced by chlorine, the dimeric [Bk (C 5 H 5 ) 2 Cl] 2 being formed. The optical absorption spectrum of this compound is very similar to (η 5 –C 5 H 5 ) 3 Bk.
safety instructions
Classifications according to the CLP regulation are not available because they only include chemical hazard and play a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
literature
- David E. Hobart and Joseph R. Peterson: Berkelium ; in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1444-1498 ( doi: 10.1007 / 1-4020-3598-5_10 ).
- Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurane: Part A 1 I, pp. 38-40; Part A 1 II, pp. 19, 326-336; Part B 1, pp. 72-76.
- The Solution Absorption Spectrum of Bk 3+ and the Crystallography of Berkelium Dioxide, Sesquioxide, Trichloride, Oxychloride, and Trifluoride , Ph.D. Thesis, Joseph Richard Peterson, October 1967, US Atomic Energy Commission Document Number UCRL-17875 (1967).
- JR Peterson, DE Hobart: The Chemistry of Berkelium ; in: Harry Julius Emeleus (Ed.): Advances in inorganic chemistry and radiochemistry , Volume 28, Academic Press, 1984, ISBN 0-12-023628-1 , pp. 29-64 ( doi: 10.1016 / S0898-8838 (08) 60204-4 , limited preview in Google Book search).
- GT Seaborg (Ed.): Proceedings of the Symposium Commemorating the 25th Anniversary of the Discovery of Elements 97 and 98 , January 20, 1975; Report LBL-4366, July 1976.
Web links
- Entry to Berkelium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
- Amanda Yarnell: Berkelium , Chemical & Engineering News, 2003.
Individual evidence
- ↑ The values of the atomic and physical properties (info box) are, unless otherwise stated, taken from: David E. Hobart and Joseph R. Peterson: Berkelium . Ed .: Lester R. Morss, Norman M. Edelstein, Jean Fuger. Springer, Dordrecht 2006, ISBN 1-4020-3555-1 , pp. 1444–1498 , doi : 10.1007 / 1-4020-3598-5_10 ( nevada.edu [PDF; accessed March 30, 2019]).
- ↑ a b c d e entry on berkelium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on berkelium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ↑ a b Hobart, Peterson (2006), p. 1482.
- ↑ a b c d G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties ; in: Nuclear Physics A , 2003 , 729 (1), pp. 3–128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ^ SG Thompson, A. Ghiorso, GT Seaborg: Element 97 ; in: Physical Review , 1950 , 77 (6), pp. 838-839 ( doi: 10.1103 / PhysRev.77.838.2 ).
- ↑ a b c S. G. Thompson, A. Ghiorso, GT Seaborg: The New Element Berkelium (Atomic Number 97) ; in: Physical Review , 1950 , 80 (5), pp. 781-789 ( doi: 10.1103 / PhysRev.80.781 ; Maschinoscript (April 26, 1950) (PDF; 1.8 MB)).
- ↑ a b Stanley G. Thompson, Glenn T. Seaborg: Chemical Properties of Berkelium ( doi: 10.2172 / 932812 ; abstract ; machine script (February 24, 1950) ).
- ↑ a b S. G. Thompson, BB Cunningham, GT Seaborg: Chemical Properties of Berkelium ; in: J. Am. Chem. Soc. , 1950 , 72 (6), pp. 2798-2801 ( doi: 10.1021 / ja01162a538 ).
- ^ SG Thompson, BB Cunningham: First Macroscopic Observations of the Chemical Properties of Berkelium and Californium , supplement to Paper P / 825 presented at the Second International Conference of Peaceful Uses Atomic Energy, Geneva, 1958.
- ^ PR Fields, MH Studier, H. Diamond, JF Mech, MG Inghram, GL Pyle, CM Stevens, S. Fried, WM Manning (Argonne National Laboratory, Lemont, Illinois) ; A. Ghiorso, SG Thompson, GH Higgins, GT Seaborg (University of California, Berkeley, California) : Transplutonium Elements in Thermonuclear Test Debris ; in: Physical Review , 1956 , 102 (1), pp. 180-182 ( doi: 10.1103 / PhysRev.102.180 ).
- ^ High Flux Isotope Reactor , Oak Ridge National Laboratory; Retrieved September 23, 2010.
- ↑ SG Thompson, A. Ghiorso, BG Harvey, GR Choppin: Transcurium Isotopes Produced in the Neutron Irradiation of Plutonium ; in: Physical Review , 1954 , 93 (4), pp. 908-908 ( doi: 10.1103 / PhysRev.93.908 ).
- ↑ LB Magnusson, MH Studier, PR Fields, CM Stevens, JF Mech, AM Friedman, H. Diamond, JR Huizenga: Berkelium and Californium Isotopes Produced in Neutron Irradiation of Plutonium ; in: Physical Review , 1954 , 96 (6), pp. 1576-1582 ( doi: 10.1103 / PhysRev.96.1576 ).
- ^ TA Eastwood, JP Butler, MJ Cabell, HG Jackson (Atomic Energy of Canada Limited, Chalk River, Ontario, Canada) ; RP Schuman, FM Rourke, TL Collins (Knolls Atomic Power Laboratory, Schenectady, New York) : Isotopes of Berkelium and Californium Produced by Neutron Irradiation of Plutonium ; in: Physical Review , 1957 , 107 (6), pp. 1635-1638 ( doi: 10.1103 / PhysRev.107.1635 ).
- ↑ Information on the Berkelium element at www.speclab.com ( Memento of the original from October 20, 2007 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. (engl.) ; Retrieved on: September 22, 2008.
- ^ EK Hulet: New Isotope of Berkelium ; in: Physical Review , 1956 , 102 (1), pp. 182-182 ( doi: 10.1103 / PhysRev.102.182 ).
- ↑ J. Milsted, AM Friedman, CM Stevens: The Alpha Half-life of Berkelium-247; a new long-lived isomer of Previous Berkelium-248 ; in: Nuclear Physics , 1965 , 71 (2), pp. 299-304 ( doi: 10.1016 / 0029-5582 (65) 90719-4 ).
- ↑ Kimberly E. Williams, Glenn T. Seaborg: New Isotope 242 Bk ; in: Physical Review C , 1979 , 19 (5), pp. 1794-1800 ( doi: 10.1103 / PhysRevC.19.1794 ).
- ↑ a b c J.R. Peterson, JA Fahey, RD Baybarz: The Crystal Structures and Lattice Parameters of Berkelium Metal ; in: J. Inorg. Nucl. Chem. , 1971 , 33 (10), pp. 3345-3351 ( doi: 10.1016 / 0022-1902 (71) 80656-5 ).
- ↑ JC Spirlet, JR Peterson, LB Asprey; in: Advances in Inorganic Chemistry , Vol. 31 (edited by HJ Emeléus and AG Sharpe), Academic Press, Orlando FL 1987, pp. 1-41.
- ↑ J. Fuger, RG Haire, JR Peterson: A New Determination of the Enthalpy of Solution of Berkelium Metal and the Standard Enthalpy of Formation of Bk 3+ (aq) ; in: J. Inorg. Nucl. Chem. , 1981 , 43 (12), pp. 3209-3212 ( doi: 10.1016 / 0022-1902 (81) 80090-5 ).
- ^ SE Nave, PG Huray, RG Haire, in: JE Crow, RP Guertin, TW Mihalisin (eds.): Crystalline Electric Field and Structural Effects in f-Electron Systems , Plenum, New York 1980, ISBN 0-306-40443- 5 , pp. 269-274.
- ↑ a b Peterson, Hobart (1984), p. 45.
- ↑ a b Hobart, Peterson (2006), p. 1460.
- ↑ Jim C. Sullivan, KH Schmidt, LR Morss, CG Pippin, C. Williams: Pulse Radiolysis Studies of Berkelium (III): Preparation and Identification of Berkelium (II) in Aqueous Perchlorate Media ; in: Inorg. Chem. , 1988 , 27 (4), pp. 597-598 ( doi: 10.1021 / ic00277a005 ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1956.
- ^ Peterson, Hobart (1984), p. 55.
- ^ Hobart, Peterson (2006), p. 1472.
- ^ Z. Assefa, RG Haire, NA Stump: Emission profile of Bk (III) in a silicate matrix: anomalous dependence on excitation power ; in: Journal of Alloys and Compounds , 1998 , 271-273 , pp. 854-858 ( doi: 10.1016 / S0925-8388 (98) 00233-3 ).
- ^ Rita Cornelis, Joe Caruso, Helen Crews, Klaus Heumann: Handbook of Elemental Speciation II: Species in the Environment, Food, Medicine & Occupational Health , John Wiley and Sons, 2005, ISBN 0-470-85598-3 , p. 552 ( limited preview in Google Book search).
- ↑ G. Pfennig, H. Klewe-Nebenius, W. Seelmann-Eggebert (eds.): Karlsruher Nuklidkarte , 7th edition, 2006.
- ↑ MB Chadwick, P. Oblozinsky, M. Herman at al .: ENDF / B-VII.0: Next Generation Evaluated Nuclear Data Library for Nuclear Science and Technology ; in: Nuclear Data Sheets , 2006 , 107 (12), pp. 2931-3060 ( doi: 10.1016 / j.nds.2006.11.001 ).
- ^ AJ Koning, et al .: The JEFF evaluated data project , Proceedings of the International Conference on Nuclear Data for Science and Technology, Nice, 2007 ( doi: 10.1051 / ndata: 07476 ).
- ↑ a b Institut de Radioprotection et de Sûreté Nucléaire : Evaluation of nuclear criticality safety data and limits for actinides in transport , p. 16 ( PDF ( Memento of November 18, 2014 in the Internet Archive )).
- ^ Finally, Item 117 Is Here! , Science Now , Retrieved April 7, 2010.
- ↑ Hobart, Peterson (2006), pp. 1445-1448.
- ^ Richard G. Haire: Californium ; in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1499-1576 ( doi: 10.1007 / 1-4020-3598-5_11 ).
- ↑ Collaboration Expands the Periodic Table, One Element at a Time ( Memento of the original from July 18, 2011 in the Internet Archive ) Info: The archive link has been inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. , Science and Technology Review, Lawrence Livermore National Laboratory, October / November 2010.
- ↑ Nuclear Missing Link Created at Last: Superheavy Element 117 , Science daily , accessed on: April 7, 2010.
- ↑ Joseph R. Peterson, Burris B. Cunningham: Some Chemical Properties of Element 97 (UCRL-17337) ; in: Trans. Amer. Nucl. Soc. , 1967 , 10 , pp. 92-93 ( [1] ).
- ↑ JR Peterson, BB Cunningham: Crystal Structures and Lattice Parameters of the Compounds of Berkelium I. Berkelium Dioxide and Cubic Berkelium Sesquioxide ; in: Inorg. Nucl. Chem. Lett. , 1967 , 3 (9), pp. 327-336 ( doi: 10.1016 / 0020-1650 (67) 80037-0 ).
- ↑ a b R. D. Baybarz: The Berkelium Oxide System ; in: J. Inorg. Nucl. Chem. , 1968 , 30 (7), pp. 1769-1773 ( doi: 10.1016 / 0022-1902 (68) 80352-5 ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1972.
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1969.
- ↑ a b D. D. Ensor, JR Peterson, RG Haire, JP Young: Absorption Spectrophotometric Study of Berkelium (III) and (IV) Fluorides in the Solid State ; in: J. Inorg. Nucl. Chem. , 1981 , 43 (5), pp. 1001-1003 ( doi: 10.1016 / 0022-1902 (81) 80164-9 ).
- ↑ JR Peterson, BB Cunningham: Crystal Structures and Lattice Parameters of the Compounds of Berkelium IV. Berkelium Trifluoride ; in: J. Inorg. Nucl. Chem. , 1968 , 30 (7), pp. 1775-1784 ( doi: 10.1016 / 0022-1902 (68) 80353-7 ).
- ↑ JR Peterson, BB Cunningham: Crystal Structures and Lattice Parameters of the Compounds of Berkelium II. Berkelium Trichloride ; in: J. Inorg. Nucl. Chem. , 1968 , 30 (3), pp. 823-828 ( doi: 10.1016 / 0022-1902 (68) 80443-9 ).
- ↑ JR Peterson, JP Young, DD Ensor, RG Haire: Absorption Spectrophotometric and X-Ray Diffraction Studies of the Trichlorides of Berkelium-249 and Californium-249 ; in: Inorg. Chem. , 1986 , 25 (21), pp. 3779-3782 ( doi: 10.1021 / ic00241a015 ).
- ↑ John H. Burns, Joseph Richard Peterson: The Crystal Structures of Americium Trichloride Hexahydrate and Berkelium Trichloride Hexahydrate ; in: Inorg. Chem. , 1971 , 10 (1), pp. 147-151 ( doi: 10.1021 / ic50095a029 ).
- ↑ John H. Burns, JR Peterson, JN Stevenson: Crystallographic Studies of some Transuranic Trihalides: 239 PuCl 3 , 244 CmBr 3 , 249 BkBr 3 and 249 CfBr 3 ; in: J. Inorg. Nucl. Chem. , 1975 , 37 (3), pp. 743-749 ( doi: 10.1016 / 0022-1902 (75) 80532-X ).
- ↑ Peterson, Hobart (1984), p. 48.
- ^ Hobart, Peterson (2006), p. 1469.
- ^ RL Fellows, JP Young, RG Haire, in: Physical-Chemical Studies of Transuranium Elements (Progress Report April 1976-March 1977) (ed. By JR Peterson), US Energy Research and Development Administration Document ORO-4447-048, University of Tennessee, Knoxville, pp. 5-15.
- ↑ a b c Peterson, Hobart (1984), p. 53.
- ^ Hobart, Peterson (2006), pp. 1465, 1470.
- ↑ a b c Hobart, Peterson (2006), p. 1470.
- ↑ a b J. N. Stevenson, JR Peterson: Preparation and Structural Studies of Elemental Curium-248 and the Nitrides of Curium-248 and Berkelium-249 ; in: Journal of the Less Common Metals , 1979 , 66 (2), pp. 201-210 ( doi: 10.1016 / 0022-5088 (79) 90229-7 ).
- ↑ D. Damien, RG Haire, JR Peterson: Preparation and Lattice Parameters of 249 Bk Monopnictides ; in: J. Inorg. Nucl. Chem. , 1980 , 42 (7), pp. 995-998 ( doi: 10.1016 / 0022-1902 (80) 80390-3 ).
- ^ Hobart, Peterson (2006), p. 1455.
- ↑ Peterson, Hobart (1984), pp. 39-40.
- ↑ Hobart, Peterson (2006), pp. 1470-1471.
- ^ Peterson, Hobart (1984), p. 47.
- ↑ a b Peterson, Hobart (1984), p. 54.
- ^ Hobart, Peterson (2006), p. 1463.
- ↑ Christoph Elschenbroich : Organometallchemie , 6th edition, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , pp. 583-584.
- ↑ Peter G. Laubereau, John H. Burns: Microchemical Preparation of Tricyclopentadienyl Compounds of Berkelium, Californium, and some Lanthanide Elements ; in: Inorg. Chem. , 1970 , 9 (5), pp. 1091-1095 ( doi: 10.1021 / ic50087a018 ).
- ↑ PG Lauberau: The formation of dicyclopentadienylberkeliumchloride ; in: Inorg. Nucl. Chem. Lett. , 1970 , 6 , pp. 611-616 ( doi: 10.1016 / 0020-1650 (70) 80057-5 ).
- ^ Hobart, Peterson (2006), p. 1471.




![\ mathrm {^ {247} _ {\ 97} Bk \ \ xrightarrow [1380 \ a] {\ alpha} \ ^ {243} _ {\ 95} on \ \ xrightarrow [7370 \ a] {\ alpha} \ ^ {239} _ {\ 93} Np \ \ xrightarrow [2,355 \ d] {\ beta ^ -} \ ^ {239} _ {\ 94} Pu \ \ xrightarrow [24110 \ a] {\ alpha} \ ^ {235 } _ {\ 92} U}](https://wikimedia.org/api/rest_v1/media/math/render/svg/ae4921d3479472c482947d24b4155288cadf4796)
![{\ mathrm {^ {{238}} _ {{\ 92}} U \ {\ xrightarrow {(n, \ gamma)}} \ _ {{\ 92}} ^ {{239}} U \ {\ xrightarrow [{23.5 \ min}] {\ beta ^ {-}}} \ _ {{\ 93}} ^ {{239}} Np \ {\ xrightarrow [{2.3565 \ d}] {\ beta ^ {-}}} \ _ {{\ 94}} ^ {{239}} Pu}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/00b6f3352024666bbfe7ef4deba05e694195bfdf)
![{\ mathrm {^ {{239}} _ {{\ 94}} Pu \ {\ xrightarrow {4 (n, \ gamma)}} \ _ {{\ 94}} ^ {{243}} Pu \ {\ xrightarrow [{4,956 \ h}] {\ beta ^ {-}}} \ _ {{\ 95}} ^ {{243}} on \ {\ xrightarrow {(n, \ gamma)}} \ _ {{\ 95}} ^ {{244}} On \ {\ xrightarrow [{10.1 \ h}] {\ beta ^ {-}}} \ _ {{\ 96}} ^ {{244}} cm}} \ quad; \ quad {\ mathrm {^ {{244}} _ {{\ 96}} cm \ {\ xrightarrow {5 (n, \ gamma)}} \ _ {{\ 96}} ^ {{249}} Cm}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/20fba3c3fea74c7ee348a8b3c4a336a79b1537cf)
![{\ mathrm {^ {{249}} _ {{\ 96}} cm \ {\ xrightarrow [{64.15 \ min}] {\ beta ^ {-}}} \ _ {{\ 97}} ^ { {249}} Bk \ {\ xrightarrow [{330 \ d}] {\ beta ^ {-}}} \ _ {{\ 98}} ^ {{249}} Cf}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/4a442eb6b9df6de89b3d39182cf49f3c45daaadc)
![{\ mathrm {^ {{249}} _ {{\ 97}} Bk \ {\ xrightarrow {(n, \ gamma)}} \ _ {{\ 97}} ^ {{250}} Bk \ {\ xrightarrow [{3,212 \ h}] {\ beta ^ {-}}} \ _ {{\ 98}} ^ {{250}} Cf}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d1ab240176cca76b16e7835a565f5b491a42cbc9)
![\ mathrm {^ {244} _ {\ 96} Cm \ \ xrightarrow [] {(\ alpha, n)} \ ^ {247} _ {\ 98} Cf \ \ xrightarrow [3.11 \ h] {\ epsilon } \ ^ {247} _ {\ 97} Bk}](https://wikimedia.org/api/rest_v1/media/math/render/svg/d143fca53ec79d59a3a77098ed208112e6be6274)
![\ mathrm {^ {244} _ {\ 96} Cm \ \ xrightarrow [] {(\ alpha, p)} \ ^ {247} _ {\ 97} Bk}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3fd61edf5e167f75669fbc6a521bd8e01211e482)