Fluorite
| Fluorite | |
|---|---|
| purple fluorite from Morocco | |
| General and classification | |
| other names |
|
| chemical formula | CaF 2 |
|
Mineral class (and possibly department) |
Halides |
|
System no. to Strunz and to Dana |
3.AB.25 ( 8th edition : III / A.08) 02/09/01/01 |
| Crystallographic Data | |
| Crystal system | cubic |
| Crystal class ; symbol | cubic hexakisoctahedral; 4 / m 3 2 / m |
| Space group | Fm 3 m (No. 225) |
| Lattice parameters | a = 5.463 Å |
| Formula units | Z = 4 |
| Frequent crystal faces | {001}, {111} |
| Twinning | Crossing twins according to (111) |
| Physical Properties | |
| Mohs hardness | 4th |
| Density (g / cm 3 ) | 3.2 |
| Cleavage | completely after {111} |
| Break ; Tenacity | shell-like to splintery |
| colour | in its pure form colorless, yellow, green, red, violet also blackish |
| Line color | White |
| transparency | transparent to opaque |
| shine | Glass gloss |
| radioactivity | sometimes by ingrown uranium minerals |
| Crystal optics | |
| Refractive index | n = 1.433 to 1.448 |
| Birefringence | no |
| Other properties | |
| Chemical behavior | is dissolved by sulfuric acid |
| Special features | blue to blue-green fluorescence, phosphorescence, triboluminescence |
Fluorite , also known under the mining name fluorspar or its chemical name calcium fluoride , is the calcium salt of hydrofluoric acid and a very common mineral from the mineral class of simple halides . Fluorite crystallizes in the cubic crystal system with the chemical composition CaF 2 and develops cubic crystals with predominantly cubic or rarely octahedral crystal form as well as interpenetrating twins , but also granular, massive aggregates .
Pure fluorite is colorless and transparent, and also gray due to impurities. However, it can take on almost all colors, mostly in weak intensity, through foreign admixtures. Green, violet to black-violet and yellow crystals ("honey spar") are common, but blue, red and brown fluorites are also found. A zonal color change can also often be observed. The line color , on the other hand, is always white.
Fluorite is the key mineral (scaling mineral) on the Mohs hardness scale for hardness 4.
Etymology and history
Fluorspar was already known in ancient Greece . The German name goes back to its use as a flux in metal processing. In 1824 the German mineralogist Friedrich Mohs discovered fluorescence that becomes visible in ultraviolet light .
The Irish mathematician and physicist George Gabriel Stokes named the phenomenon of fluorescence after the fluorite in analogy to the opalescence of the opal .
classification
In the outdated, but partly still in use 8th edition of the mineral classification according to Strunz , fluorite belonged to the general division of "simple halides", where together with coccinite , frankdicksonite , gagarinite (Y) , laurelite , tveitite (Y) and gagarinite - (Ce) (formerly Zajacit- (Ce) ) formed its own group.
The 9th edition of Strunz's mineral systematics , which has been in effect since 2001 and is used by the International Mineralogical Association (IMA), classifies fluorite in the new and more precise division of "Simple halides without H 2 O". This is further subdivided according to the molar ratio of cations (M) and anions (X), so that the mineral can be found in the sub-section "M: X = 1: 2" according to its composition, where it is named after the "fluorite group" with the system no. 3.AB.25 and the other members fluorocronite ( IMA 2010-023 ), frankdicksonite, strontiofluorite ( IMA 2009-014 ).
The systematics of minerals according to Dana , which is common in the English-speaking world , assigns fluorite to the class of "halides (and relatives)" and there in the department of "halides". Here it is the eponymous mineral of the "fluorite group" with the system no. 09.02.01 and the other members Frankdicksonit, Tveitit- (Y) and Strontiofluorit within the sub-section of " Anhydrous and hydrous halides with the formula AX 2 ".
Crystal structure
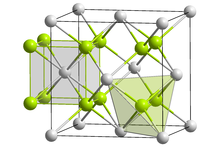
Fluorite crystallizes in the cubic crystal system in the highly symmetrical crystal class 4 / m 3 2 / m ( cubic-hexakisoctahedral ) or the space group Fm 3 m (space group no. 225) with the lattice parameter a = 5.463 Å and 4 formula units per unit cell .
In the crystal structure , the Ca 2+ ions form a cubic closest packing of spheres , which corresponds to a face-centered cubic lattice (fcc, face centered cubic ). The surface centering of the unit cell (see picture on the right) can also be read from the room group symbol (" F "). The fluoride ions (F - ) occupy all the tetrahedral gaps in the densest packing of calcium ions. Since a close packing of spheres always contains twice as many tetrahedral gaps as packing particles, the structure has a calcium-fluorine ratio of 1: 2, which is also reflected in the chemical formula of fluorite, CaF 2 . The coordination polyhedron for the fluoride ions is a tetrahedron made up of four calcium ions, while the calcium ions are surrounded by eight fluorine atoms in the form of a cube . The cation and anion sublattices are not commutative; H. interchangeable. The so-called fluorite structure is found in a number of other salts, such as the fluorides SrF 2 , BaF 2 , CdF 2 , HgF 2 and PbF 2 . The fluorite structure also occurs in Li 2 O, Li 2 S, Na 2 O, Na 2 S, K 2 O, K 2 S, Rb 2 O and Rb 2 S, for example . Its crystal structure is isotypic with uraninite .
properties
morphology
Fluorite often forms well-formed, cube-shaped and octahedral crystals. In combination with these main shapes, fluorite crystals often show surfaces of other shapes. The areas of the rhombic dodecahedron {110}, the tetrakis hexahedron {210} (additional areas parallel to the cube edges), the ikositetrahedron {211} or {311} and the hexakiso octahedron {421} are widespread .
The crystal habit of the fluorite crystals is temperature-dependent. At high temperatures, octahedra {111} are formed, rhombic dodecahedra {110} at medium temperatures, and cubes {100} as the dominant forms at low temperatures.
The faces of the cube are usually smooth and shiny. Octahedral and rhombic dodecahedron surfaces, on the other hand, often appear rough and matt and are then usually composed of tiny cube surfaces. The loose, octahedron-shaped fluorite crystals with smooth, shiny surfaces that are widespread in the trade are almost never crystals that have grown in this form, but rather split octahedra.
Furthermore, fluorite forms spherical and grape-shaped aggregates, crusts or stalactites. The scalenohedron fluorite is a specialty, as is shown by finds from the Cäcilia fluorspar mine near Freiung (municipality of Stulln in the Upper Palatinate ). On closer examination, however, it turned out that these also had to be assigned to the cubic system and were only caused by chemical burns.
- Crystal forms and aggregates
Cube and octahedron ( cuboctahedron ) {100}, {111}
Spherical fluorite
Physical Properties

Due to the incorporation of lanthanides , for example Eu 2+ , fluorite can show strong fluorescence when excited with UV light and, when heated, also phosphorescence and triboluminescence under high mechanical stress .
Fluorite is an electrical insulator . Its melting point is 1392 ° C.
In the thin section under the microscope, fluorite is noticeable in linearly polarized light because, due to its relatively low refractive index, it shows a strongly negative relief compared to almost all accompanying minerals. Under crossed polarizing filters, it shows isotropy as a cubic crystallized mineral, ie it remains dark.
Chemical properties
In case of contact with strong acids such as B. sulfuric acid , fluorite releases highly toxic hydrogen fluoride .
colour
Although pure CaF 2 is colorless, fluorite is one of the minerals with the most color variations. The dark color of many fluorites is caused by embedded rare earths or radioactive irradiation of the fluorspar ( stinkspar ), whereby ingrown uranium minerals can also intensify the color.
The causes of the color are diverse and not always fully clarified. Mostly trace amounts of rare earth elements have a coloring effect , which are often only ionized by radioactive radiation to become colored ions. Which rare earth elements are ionized during this process can depend on the type of radiation. For example, with the same trace element content, fluorites can develop different colors in the vicinity of thorium-containing minerals than in the vicinity of uranium-containing minerals. Furthermore, the temperature history can influence the color as well as the incorporation of oxygen ions and OH - or other coloring ions. Trace contents of non-coloring ions such as Na + or Fe 3+ stabilize coloring lattice defects and thus also influence the color.
- Yellow: The color of yellow fluorites is based on the incorporation of O 3 - and O 2 - instead of two neighboring F - ions. The charge is balanced by replacing Ca 2+ with Na + .
- Light green: The light green color of many fluorites is based on trace levels of Sm 2+ . Samarium (Sm) is incorporated as Sm 3+ instead of Ca 2+ . The reduction to Sm 2+ takes place through the uptake of an electron, which is released when other cations are oxidized by ionizing radiation .
The stability of the green color depends on which cations are oxidized. Fluorites formed under reducing conditions contain traces of Fe 2+ , which can be oxidized to Fe 3+ and, together with samarium, produce a temperature-stable green color. In fluorites formed under more oxidizing conditions, the green-coloring Sm 2+ ions are generated and stabilized by the oxidation of Ce (Pr, Tb) 3+ to Ce (Pr, Tb) 4+ . Fluorites that are colored by this mechanism will fade in daylight or when heated.
- Yellow-green: Fluorites of some localities (Redruth in England, Tübingen in Germany) show a green to yellow-green color, which indicates the common occurrence of traces of yttrium (Y 3+ ) and cerium (Ce 3+ ) each with a color center (blank on one F - position, in which there are two electrons) in the immediate vicinity.
- Light blue: Fluorites that contain only Y 3+ , together with a space on an F - position in which there are two electrons, are colored light blue.
- Dark blue: Synthetic fluorites can be colored intensely blue to purple due to the formation of colloidal calcium (metallic). The blue-violet color of the naturally occurring "Blue John Fluorite" from Castelton near Derbyshire in England is also attributed to it.
- Violet: The cause of the widespread violet coloration of natural fluorites has not been conclusively clarified. Currently, electron defects in the crystal lattice are the most likely cause of the purple color of fluorite.
- Pink, red: The pink to red color of fluorites is caused by the incorporation of O 2 3− molecules, which are stabilized by neighboring Y 3+ ions.
- Color variations
Stinkspar (antozonite)
Stinkspat is a dark purple to black variety of fluorite that develops a pungent odor when chopped up. Stinkspar often (but not always) occurs together with uranium minerals, some of which can be included in the stinkspar as very fine particles. The type locality and best known German site is Wölsendorf in the Upper Palatinate .
Rubbing or hitting the crystal releases gaseous, toxic fluorine (F 2 ), which causes the odor.
The dark purple to black color has several causes. Colloidal metallic calcium plays a major role, which leads to a dark blue to black color. In addition, there are free electrons on empty fluorine positions (F centers), which are typical of purple fluorite.
All of these properties of stinkspate are due to the radioactive irradiation of the fluorite. Stinkspar typically occurs with uranium-containing minerals. The uranium and thorium contained therein decays and emits gamma radiation. This radiation releases electrons from the F - ions and an H-center is formed, a neutral fluorine atom on an otherwise empty lattice position, which forms an atomic bond to a neighboring F - ion. The released electrons are trapped by lattice defects, empty fluorine positions, and form F centers there , individual electrons on an F - position, which are surrounded by 4 Ca 2+ ions. These F-Centers are not stationary. They diffuse through the crystal lattice and combine with other F centers to form fluorine-free Ca nanoparticles with a diameter of 5 to 30 nm. These clusters are also known as "colloidal Ca" and contribute to the blue-black color of the stinkspate.
Varieties
So far, several varieties of fluorite are known in which small amounts of calcium have been replaced by rare earths such as cerium and yttrium :
Yttrofluorite (1911) and cerium fluorite were initially described by Thorolf Vogt as new mineral types from northern Norway. After more extensive melt flow analyzes by Gustav Tammann and Vogt in 1913 , it was found that fluorite can contain up to 50% by weight yttrium fluoride (YF 3 , yttrofluorite ) and up to 55.8% cerium (III) fluoride (CeF 3 , cerium fluorite ) . Theoretically there is therefore a three-component mixed crystal system , although the idealized compositions YF 3 and CeF 3 have so far only been known synthetically and have only been investigated up to the above-mentioned percentages of fluorite.
Neither cerium fluorite nor yttrofluorite have so far been discovered in nature with a substance purity that is sufficiently high for recognition by the International Mineralogical Association (IMA). Yttrofluorite was officially discredited by the IMA in 2006, whereas cerfluorite was listed as a hypothetical mineral in the IMA mineral list until 2009.
Yttrocerite was described as a mineral by Johan Gottlieb Gahn and Jöns Jakob Berzelius in 1815 ; However, Vogt stated in his analysis results in 1913 that this was a mixture of yttrofluorite and cerium fluorite .
Education and Locations
Fluorite is usually massive, occasionally also in crystalline form, and is often associated with barite , quartz , topaz , calcite , galena and zinc blende . Hydrothermal veins, in which fluorite forms the vein, represent the commercially most important type of its occurrence. In addition, deposits formed by metasomatosis are important, whereby synsedimentary formation is also considered for some deposits in sedimentary rocks. Otherwise, it is rare as a rock-forming mineral and is only occasionally found as a secondary part in fluorine-containing old men , as well as in granites , carbonatites and other igneous rocks such as pegmatites .
The world's largest fluorspar mining is found in Mexico, in the Las Cuevas deposit, which is of volcanic origin. Other rich fluorspar deposits are in China, in the Indian Amba Dongar , in South Africa ( Zwartkloof and Witkop in the Transvaal ), in Namibia (Okorusu), in the Kenyan Kario Valley and in the US states of Illinois and Kentucky . A well-known find of fluorspar in Europe are the mountains and caves around Castleton , in the English Peak District , where it is known under the name "Blue John" and is mined for jewelry making. The name is a corruption of the French "bleu et jaune", which means something like "blue-yellow".
German deposits are, for example, the Clara mine near Oberwolfach in the Black Forest and the Käfersteige mine near Pforzheim ; In addition, fluorite is also found in the Upper Palatinate , between Nabburg and Schwarzenfeld , in the Schortetal near Ilmenau in the Thuringian Forest (see also: Volle Rose show mine ), in the Ore Mountains near Freiberg and Bärenstein , in the Vogtland in Schönbrunn (Vogtland river spar works or Patriot) and in eastern Harz near Straßberg. It was found in small quantities in many mineral deposits in Germany. In the 1970s an occurrence in Zechstein dolomites in the Eschwege - Sontra area in northern Hesse was investigated, but these activities were discontinued due to fluctuations in the world market.
In Austria, for example, the Vorderkrimml deposit - now exhausted - is known, today a show tunnel.
use
As a raw material
Fluorite is mainly used industrially
- as Hüttenspat in the metal industry as a flux for slag in the metallurgical process , particularly as a supplement in the open hearth furnace and electric furnace , and for the production of artificial cryolite for aluminum production ,
- as acid spar for the production of fluorine and hydrofluoric acid as well as various fluorides or secondary products such as fluorocarbons and polymeric fluorine compounds (e.g. polytetrafluoroethylene ),
- as ceramic spatula in the glass industry as a flux and opacifier for z. B. frosted glass , frosted glass and opalescent glasses, for ceramic materials and as a base material for optical lenses (CaF 2 single crystals, fluoride glasses based on beryllium fluoride , fluorite, and sodium fluoride ). The ability to break the light spectrum evenly allows the chromatic aberration of lenses to be compensated. The problem here is that particularly large crystals are required for high-performance lenses; these are artificially grown. Crystals of this size have the property of being warped by heat (solar radiation) in such a way that they significantly change the calculation of the optics.
As a gem
Due to its rather low hardness, fluorite is of little interest as a gemstone for the commercial jewelry industry . Occasionally it is used by glyptists and hobby cutters to make small, craft objects or faceted gemstones .
But since it can be confused with many precious stone minerals due to its variety of colors, it often serves as the basis for imitations . To change the colors, fluorite is either fired or irradiated. To protect against damage or to cover up damage caused by cracks, fluorite gemstones are often stabilized with synthetic resin (see also Gemstone ).
See also
literature
Monographs:
- Gerhard Niedermayr: Mineral of the rainbow: fluorite . Doris Bode Verlag, Haltern 1990, ISBN 3-925094-44-X .
- HG Dill, B. Weber: The Upper Palatinate fluorspar anthology - "Colorful stones" shape the region and its people around the Wölsenberg . Druckkultur Späthling, Weißenstadt, 2011, ISBN 978-3-942668-01-9 .
In compendia:
-
Walter Ehrenreich Tröger u. a. (Ed.): Optical determination of rock-forming minerals , Swiss beard, Stuttgart
- 1. - Determination tables , 1982, ISBN 3-510-65106-5
- Martin Okrusch, Siegfried Matthes: Mineralogy . 7th edition. Springer Verlag, Berlin 2005, ISBN 3-540-23812-3
- Petr Korbel, Milan Novák: Encyclopedia of Minerals . Nebel Verlag GmbH, Eggolsheim 2002, ISBN 3-89555-076-0 , p. 73 .
- Walter Schumann: Precious stones and gemstones . 13th edition. BLV Verlags GmbH, 1976/1989, ISBN 3-405-16332-3
Scientific articles:
- H. Bill, G. Calas: Color centers, associated rare-earth ions and the origin of coloration in natural fluorites . In: Physics and chemistry of minerals . No. 3 , 1978, p. 117-131 , doi : 10.1007 / BF00308116 .
- G. Bayer, HG Wiedemann: Fluorine raw materials - occurrence, use and problems, chemistry in our time, 19th year 1985, No. 2, pp. 35-41, doi : 10.1002 / ciuz.19850190202
- U. Kempe, M. Plötze, A. Brachmann and R. Böttcher: Stabilization of divalent rare earth elements in natural fluorite . In: Mineralogy and Petrology . No. 76 , 2002, p. 213-234 , doi : 10.1007 / s007100200042 .
- U. Kempe: On Violet Coloration of Natural Fluorite . RMS DPI, 2006, p. 162–164 ( Online [PDF; 139 kB ]).
- HG Dill, B. Weber: Variation of color, structure and morphology of fluorite and the origin of the hydrothermal F-Ba deposits at Nabburg-Wölsendorf, SE Germany . New Yearbook for Mineralogy - Treatises, 2010, p. 113–132 ( summary [PDF; 45 kB ]).
Web links
- Mineralienatlas: Fluorit (technical information), Mineralienatlas: Mineralienportrait / Fluorit (comprehensive portrait)
- Virtual tour Flußspatgang Reichhartschacht
- MinDat - Fluorite (English)
- Webmineral (English)
Individual evidence
- ^ A b Hugo Strunz , Ernest H. Nickel : Strunz Mineralogical Tables. Chemical-structural Mineral Classification System . 9th edition. E. Schweizerbart'sche Verlagbuchhandlung (Nägele and Obermiller), Stuttgart 2001, ISBN 3-510-65188-X , p. 153 .
- ^ GG Stokes: On the Change of Refrangibility of Light . In: Philosophical Transaction Royal Society London . tape 142 , 1852, pp. 463-562 ( available online at archive.org - Internet Archive [accessed July 31, 2017]).
- ^ Helmut Schrätze , Karl-Ludwig Weiner : Mineralogie. A textbook on a systematic basis . de Gruyter, Berlin; New York 1981, ISBN 3-11-006823-0 , pp. 322-327 .
- ^ Mineral Atlas : Cäcilia Pit. Retrieved July 31, 2017 .
- ↑ a b Hans Jürgen Rösler : Textbook of Mineralogy . 4th revised and expanded edition. German publishing house for basic industry (VEB), Leipzig 1987, ISBN 3-342-00288-3 , p. 355-356 .
- ↑ Hans Pichler, Cornelia Schmitt-Riegraf: Rock -forming minerals in thin sections . 2nd Edition. Enke, Stuttgart 1993, ISBN 3-8274-1260-9 , pp. 50 .
- ↑ a b U. Kempe: On Violet Coloration of Natural Fluorite . RMS DPI, 2006, p. 162–164 ( PDF 139 kB ( Memento from September 7, 2016 in the Internet Archive )).
- ↑ a b U. Kempe, M. Plötze, A. Brachmann, R. Böttcher: Stabilization of divalent rare earth elements in natural fluorite . In: Mineralogy and Petrology . tape 76 , 2002, p. 213-234 , doi : 10.1007 / s007100200042 .
- ↑ a b c d e f H. Bill, G. Calas: Color centers, associated rare-earth ions and the origin of coloration in natural fluorites . Physics and chemistry of minerals Vol. 3, 1978, pp. 117-131 , doi : 10.1007 / BF00308116 .
- ↑ Jörn Schmedt on the Günne, Martin Mangstl, Florian Kraus: Elementary fluorine F 2 in nature - in-situ detection and quantification by NMR spectroscopy . In: Angewandte Chemie . tape 124 , 2012, p. 7968–7971 , doi : 10.1002 / anie.201203515 .
- ↑ Marten Huisinga: Ultraviolet photoelectron spectroscopy and electron stimulated desorption from CaF2. 1999, accessed July 31, 2017 .
- ↑ Thorolf Vogt: Preliminary communication about yttrofluorite, a new mineral species from northern Norway . In: Chemisches Centralblatt . tape 2 , no. 5 , August 2, 1911, p. 299–300 ( Online [PDF; 11.2 MB ; accessed on July 31, 2017]).
- ↑ a b Thorolf Vogt: About the fluorspar-yttrofluorite group . In: M. Bauer, Fr. Frech, Th. Liebisch (Hrsg.): New yearbook for mineralogy, geology and paleontology . tape 2 . Schweizbart, Stuttgart 1914, p. 9–15 ( available online at archive.org - Internet Archive [accessed July 31, 2017]).
- ↑ IMA / CNMNC List of Mineral Names 2009. (PDF; 1.9 MB) Accessed July 31, 2017 (pp. 49 and 310).
- ^ Johan Gottlieb Gahn, Jöns Jakob Berzelius: Afhandlingar I Fysik . In: Kemi och Mineralogi . tape 4 . Stockholm 1815, p. 161 ( online [PDF; 3.7 MB ; accessed on July 31, 2017]).
- ^ Walter L Pohl: Mineral and energy raw materials . 5th edition. Schweizerbart, Stuttgart 2005, ISBN 978-3-510-65212-9 , p. 252-256 .
- ^ Sächsische Zeitung: New mine in the Ore Mountains. September 10, 2009, accessed July 31, 2017 .
- ^ Sächsische Zeitung: Ore mining begins again in the Ore Mountains. October 27, 2010, accessed July 31, 2017 .
- ^ H. Ziehr, K: Matzke, E. Sawary: Flußspat in the Zechsteindolomit near Eschwege, Hessen . In: Association of Friends of Mineralogy and Geology (Hrsg.): Der Aufschluss . Special volume 28. Heidelberg 1978, p. 248-259 .
- ↑ realgems.org - fluorite (with pictures of different gemstone cuts in fluorite). Retrieved July 31, 2017 .
- ↑ Bernhard brother embellished stones. Recognizing imitations and manipulations in gemstones and minerals . Neue Erde Verlag, 2005, ISBN 3-89060-079-4 , p. 67 .