Europium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Europium, Eu, 63 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Lanthanoids | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | La , 6 , f | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white | |||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-53-1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-161-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.328 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.099 ppm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 151.964 (1) u | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 185 (231) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 198 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Xe ] 4 f 7 6 s 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 5.670 385 (5) eV ≈ 547.11 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 11.240 (6) eV ≈ 1 084.5 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 24.84 (3) eV ≈ 2 400kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 42.94 (11) eV ≈ 4 140 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 63.2 (4) eV ≈ 6 100 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | body-centered cubic | |||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 5.245 g / cm 3 (25 ° C ) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 0.013) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1099 K (826 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 1713 K (1440 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 28.97 10 −6 m 3 mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 176 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 9.2 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 1.11 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 14 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 2, 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −1.99 V (Eu 3+ + 3 e - → Eu) | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||
Europium is a chemical element with the element symbol Eu and the atomic number 63. In the periodic table it is in the group of lanthanoids and is therefore also one of the rare earth metals . Only europium and americium are elements named after a continent .
Like the other lanthanides, europium is a shiny silver heavy metal . The properties of europium do not follow the lanthanide contraction . Due to its electron configuration , the element has a significantly lower density and a lower melting and boiling point than the neighboring elements. It is the most chemically reactive rare earth metal. After initial references to the element by William Crookes and Paul Émile Lecoq de Boisbaudran , Eugène-Anatole Demarçay was able to detect the element spectroscopically in 1896 and then isolate it.
Europium is of great technical importance in phosphors , such as those used in cathode ray tube screens , which were previously used for computer monitors and televisions , in fluorescent lamps and in plasma screens . Both the red and the blue luminescent material in these screens and illuminants are substances that are doped with europium and thus show fluorescence in the corresponding spectral range .
history
A first reference to the element later called europium was found by William Crookes in 1885 . When examining the fluorescence spectra of samarium - yttrium mixtures, he was able to measure signals of an unusual orange-colored spectral line that was stronger in mixtures of the elements than in the pure substances. He called this spectral line, which points to an unknown element, the “abnormal line”, the hypothetical element S δ . Another discovery on the way to the unknown element was made in 1892 by Paul Émile Lecoq de Boisbaudran when he discovered three previously unknown blue spectral lines in the spark spectrum of Samarium in addition to the abnormal Crookes line. In 1896, Eugène-Anatole Demarçay postulated the existence of a previously unknown element between samarium and gadolinium based on ultraviolet spectra , and in 1900 he recognized that this element must be the same as that of Crookes and Boisbaudran. In 1901 Demarçay succeeded in isolating this by fractional crystallization of the samarium / europium magnesium nitrate double salts. He named the element Europium after the continent of Europe . In 1948, in analogy to europium, Glenn T. Seaborg , Ralph A. James and Leon O. Morgan named the actinoid, which is located directly below europium in the periodic table, also after a continent americium .
The first important technical application of the element was the production of europium -doped yttrium vanadate . This red phosphor , discovered in 1964 by Albert K. Levine and Frank C. Palilla , soon played an important role in the development of color television . For this application, the first mine for the extraction of rare earths, which had been in operation in the California Mountain Pass since 1954 , was expanded considerably.
Occurrence
Europium is a rare element on earth, its abundance in the continental crust is around 2 ppm .
Europium occurs as a minor component in various lanthanide minerals, minerals with europium as the main component are unknown. The element is contained in cerite earths such as monazite and bastnesite as well as in ytter earths such as xenotime , the proportion of europium is usually between 0.1 and 0.2%. The most important deposit for the extraction of europium was the Bastnäsite ore in Mountain Pass , California until 1985 , after which Chinese mines - especially the ore deposit in Bayan Obo - gained great importance.
In some igneous rocks , the concentration of europium is higher or lower than would be expected from the relative abundance ratio of the rare earth metals determined using chondrites as a standard. This phenomenon is known as the europium anomaly and is based on the fact that Eu 3+ can be reduced to Eu 2+ under reducing conditions in magma . This has a larger ionic radius than trivalent europium and is therefore easily incorporated into certain minerals, for example instead of strontium or calcium in potassium feldspar and plagioclase , which thus have a positive europium anomaly. These minerals crystallize out of the magma melt and are thereby separated, while trivalent europium remains dissolved in the residual melt. In contrast , the Eu 2+ ion is too large for incorporation into mafic rocks such as pyroxene and olivine instead of iron, magnesium and calcium and a negative europium anomaly occurs. Apart from the crystallization of plagioclase, a europium anomaly can also arise when rocks are melted. Since the distribution coefficient between crystal and melt is about 10 times greater than for the other rare earth elements, when a rock rich in plagioclase is partially melted, only a small amount of europium is released into the melt, and when it re-solidifies, a rock with a negative europium anomaly results. The europium anomaly is an indicator of the degree of fractionation of igneous rock.
A pronounced europium anomaly was found in lunar rocks , with the plagioclase-rich rocks of the moon highlands having a positive (increased europium content ), the basalt rocks found in craters and Maria a negative europium anomaly. This allows conclusions to be drawn about the geological history of the moon. It is assumed that the highlands with their anorthosites differentiated from the lunar mantle about 4.6–4.4 billion years ago and that this consists of europium-depleted olivine-pyroxene rocks. The younger basalts in the Maria, which consist of basaltic partial melts of this mantle, are therefore so poor in europium.
Extraction and presentation
Due to the similarity to the accompanying metals and the low concentration in the ores, the separation from the other lanthanides is difficult, but at the same time it is technically particularly important because of the use of the element. After digestion of the starting materials such as monazite or bastnäsite with sulfuric acid or sodium hydroxide solution , various ways of separation are possible. In addition to the ion exchange , a process is mainly used that is based on liquid-liquid extraction and the reduction of Eu 3+ to Eu 2+ . In the case of bastnäsite as a starting material, the cerium is first separated in the form of cerium (IV) oxide and the remaining rare earths are dissolved in hydrochloric acid. Then, with the help of a mixture of DEHPA (di (2-ethylhexyl) phosphoric acid) and kerosene, europium, gadolinium and samarium are separated from the other rare earth metals in liquid-liquid extraction . These three elements are separated by reducing the europium to Eu 2+ and precipitating it as poorly soluble europium (II) sulfate , while the other ions remain in solution.
Metallic europium can be obtained by reacting europium (III) oxide with lanthanum or mischmetal . If this reaction is carried out in a vacuum, europium is distilled off and can be separated from other metals and impurities:
In 2010 around 600 tons of europium were produced and 500 tons were consumed (each calculated as europium oxide). Due to the increasing demand for europium, however, it is to be feared that in the medium term demand will exceed supply and there will be a shortage. Work is therefore being carried out on expanding europium production, in particular by opening further mines such as the one in Mount Weld, Australia, and reopening the Mountain Pass mine . Due to the high demand for europium, the price of the element has also risen sharply. While in 2002 it was still at 240 US dollars per kilogram, in 2011 it rose to 1,830 dollars per kilogram (99% purity in each case).
properties
Physical Properties
Like the other lanthanoids, europium is a silvery, soft heavy metal . It has an unusually low density of 5.245 g / cm 3 , which is significantly lower than that of the neighboring lanthanides such as samarium or gadolinium and less than that of lanthanum. The same applies to the relatively low melting point of 826 ° C and the boiling point of 1440 ° C (gadolinium: melting point 1312 ° C, boiling point 3000 ° C). These values oppose the otherwise applicable lanthanide contraction and are caused by the electron configuration [Xe] 4f 7 6s 2 of europium. Due to the half-filled f-shell, only the two valence electrons (6s 2 ) are available for metallic bonds ; therefore there are lower binding forces and a significantly larger metal atom radius. The same can be observed for ytterbium . With this element, due to a completely filled f-shell, only two valence electrons are available for metallic bonds.
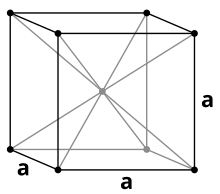
Europium crystallizes under normal conditions in a body-centered cubic lattice with the lattice parameter a = 455 pm . In addition to this structure, two other high-pressure modifications are known. The sequence of modifications with increasing pressure does not correspond to that of the other lanthanoids, as is the case with ytterbium. Neither a europium modification in a double-hexagonal structure nor in a samarium structure is known. The first phase transition in the metal takes place at 12.5 GPa, above this pressure europium crystallizes in a hexagonal, densest structure with the lattice parameters a = 241 pm and c = 545 pm. Above 18 GPa, Eu-III was found to be another structure similar to the hexagonal closest packing of spheres.
At high pressures of at least 34 GPa, the electron configuration of the europium in the metal changes from bivalent to trivalent. This also allows for superconductivity of the element at a pressure of about 80 GPa and a temperature of about 1.8 K occurs.
Europium ions that are built into suitable host lattices show a pronounced fluorescence . The emitted wavelength depends on the oxidation level. Eu 3+ fluoresces largely independently of the host lattice between 613 and 618 nm, which corresponds to an intense red color. The maximum emission of Eu 2+ , on the other hand, is more dependent on the host lattice and is, for example , 447 nm in the blue spectral range for barium magnesium aluminate and in the green spectral range for strontium aluminate (SrAl 2 O 4 : Eu 2+ ) at 520 nm .
Chemical properties

Europium is a typical base metal and reacts with most non-metals . It is the most reactive of the lanthanides and reacts quickly with oxygen. If it is heated to around 180 ° C, it ignites spontaneously in the air and burns to form europium (III) oxide.
Europium also reacts with the halogens fluorine , chlorine , bromine and iodine to form trihalides. In the reaction with hydrogen , non-stoichiometric hydride phases are formed, with the hydrogen entering the gaps in the spherical packing of the metal.
Europium dissolves slowly in water and rapidly in acids with the formation of hydrogen and the colorless Eu 3+ ion. The likewise colorless Eu 2+ ion can be obtained in an aqueous solution by electrolytic reduction on cathodes . It is the only divalent lanthanide ion that is stable in aqueous solution. Europium dissolves in ammonia , forming a blue solution, as with alkali metals, in which there are solvated electrons .
Luminescence
In addition to Sm 3+ , Tb 3+ and Dy 3+ , the Eu 3+ cation is one of the lanthanide cations which, in a suitable complex, can emit light in the visible range when certain wavelengths are absorbed. The trivalent europium cation is colorless in an aqueous solution, but if organic ligands are coordinated with an extensive π-electron system, the antenna effect ensures that the luminescent properties of the central particle increase sharply. The π-electrons of the ligand conduct the absorbed energy of the incident light (approx. 355 nm) to the 5d-electrons of the Eu 3+ , whereby these get into the 4f-orbital and when they fall back light in the visible range (at approx. 610 nm).
Isotopes
A total of 38 isotopes and a further 13 core isomers of europium between 130 Eu and 167 Eu are known. Of these, one, 153 Eu, is stable, another, 151 Eu, has long been considered stable; In 2007, however, indications were found that it decays as an alpha emitter with a half-life of at least 1.7 trillion years . These two isotopes occur in nature, 153 Eu with a share of 52.2% in the natural isotopic composition being the more common, the proportion of 151 Eu is accordingly 47.8%.
Several Europiumisotope as 152 Eu, 154 Eu and 155 Eu arise in nuclear fission of uranium and plutonium . Here, 155 Eu in a proportion of about 0.03% of the total amount of the fission products , the most common Europiumisotop under the cleavage products. It could be detected , among other things, in the Rongelap -Atoll three years after the contamination by the Castle Bravo nuclear weapons test.
use
Europium is mainly used as a dopant for the production of phosphors , which are used, for example, in cathode ray tube screens , which were previously mainly used for computer screens and televisions , as well as for aircraft instruments, and in compact fluorescent lamps . Phosphors with both bivalent and trivalent europium are used for different colors. For red phosphors, yttrium oxide doped with europium (Y 2 O 3 : Eu 3+ ) is primarily used ; yttrium oxysulfide or, as the first important red phosphor, yttrium vanadate : Eu 3+ were also used in the past . Eu 2+ is mostly used as a blue phosphor in compounds such as strontium chlorophosphate (Sr 5 (PO 4 ) 3 Cl: Eu 2+ , strontium chloroapatite SCAP) and barium magnesium aluminate (BaMgAl 11 O 17 : Eu 2+ , BAM). Plasma picture screens require phosphors that convert the VUV radiation emitted by the noble gas plasma into visible light. For this purpose, europium-doped phosphors are used for both the blue and red spectrum - BAM for blue light, BO 3 : Eu 3+ for red (Y, Gd) .
In high- pressure mercury lamps , such as those used in street lighting, europium-doped yttrium vanadate is applied to the glass so that the light appears white and more natural.
Due to its neutron absorption, europium can be used in control rods for nuclear reactors . Control rods containing europium were tested in various Soviet test reactors such as BOR-60 and BN-600 .
As EuropiumHexaBorid, it is also offered as a coating for the production of oxide cathodes for glow emission .
fluorescence
In euro banknotes europium fluorescence is used against counterfeiting.
This property can also be used in fluorescence spectroscopy . For this purpose, the europium is bound in a suitable complex , for example , which reacts preferentially at the desired location, for example with a certain protein , and accumulates there.
Biological significance and toxicity
Europium occurs only in minimal amounts in the body and has no biological significance. The element cannot be absorbed by plant roots either.
Soluble europium compounds are slightly toxic; an LD 50 value of 550 mg / kg for intraperitoneal and 5000 mg / kg for oral administration to mice was determined for europium (III) chloride . No chronic toxicity could be determined, which may be related to the low uptake of europium in the intestine and the rapid conversion of soluble europium chloride to insoluble europium oxide under basic conditions. Insoluble europium compounds are considered to be largely non-toxic, as in a study of europium (III) hydroxide - nanoparticles was determined in mice.
In europium (III) hydroxide nanoparticles (but not in amorphous europium (III) hydroxide) one was pro-angiogenic effect observed, promote in vitro the proliferation of endothelial cells , in vivo in chicken eggs increased formation of small blood vessels was observed . A possible mechanism for this observation is the formation of reactive oxygen species and the activation of MAP kinases by these nanoparticles.
links
Compounds in the oxidation states +2 and +3 are known, whereby, as with all lanthanides, although the trivalent state is the more stable, the divalent state is also unusually stable and therefore a large number of Eu (II) compounds exist. The ionic radii differ depending on the oxidation level, with Eu 2+ ions being larger than Eu 3+ ions. With the coordination number six, they are 131 pm for Eu 2+ and 108.7 pm for Eu 3+ . The effective ion radius (which uses an O 2− ion which is 140 pm larger by 14 pm as a reference ) is accordingly 117 pm or 94.7 pm for the coordination number six. The ionic radii are larger in higher coordination numbers; for Eu 2+ in the coordination number eight it is 139 pm.
Oxygen compounds
Europium (III) oxide , Eu 2 O 3 , is the technically most important europium compound and is used as a starting material for the production of other europium compounds and as a dopant for fluorescent dyes such as Y 2 O 3 : Eu 3+ , which produces a particularly intense red fluorescence with a europium ( III) oxide content of about 10%. Like the other lanthanoid oxides, it crystallizes in the cubic lanthanoid C structure.
Europium (II) oxide , EuO, is a purple-black ferromagnetic solid with a Curie temperature of 70 K that crystallizes in a sodium chloride structure. It can be obtained by reducing europium (III) oxide with europium and is the only divalent oxide of the lanthanoids that is stable under normal conditions. In addition to these two oxides, the mixed-valence oxide europium (II, III) oxide , Eu 3 O 4 , is also known.
Other europium compounds
Eu chalcogenides ( i.e. sulfides , selenides and tellurides ) and their disordered alloys have similar properties to EuO . Eu 1-x Sr x S is e.g. B. for x = 0 a ferromagnet that becomes an insulating spin glass , which is particularly suitable for computer simulations because of its non-metallic behavior.
Europium reacts with the halogens fluorine , chlorine , bromine and iodine to form the trihalides. These decompose when heated to the dihalides and elemental halogens.
Europium forms organometallic compounds. In contrast to the other lanthanides, however, no cyclopentadienyl compound of trivalent europium can be synthesized. A compound is known that contains three molecules of cyclopentadienyl and one molecule of tetrahydrofuran , but this is strongly bound to the europium and cannot be removed by heating or in a vacuum, since the compound decomposes beforehand. In contrast, europium dicyclopentadienyl (Cp) 2 Eu (II) and other known derivatives are stable. Alkynyl - europium compounds are also known from divalent europium .
The category: Europium connection offers an overview of europium connections .
literature
- Ian McGill: Rare Earth Elements. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2012, doi: 10.1002 / 14356007.a22_607 .
Web links
- Entry on europium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (Europium) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e entry on europium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on europium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1579.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics. CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ a b Entry on europium in the GESTIS substance database of the IFA , accessed on April 26, 2017(JavaScript required) .
- ^ William Crookes: On Radiant Matter Spectroscopy. Part II. Samarium. In: Philosophical Transactions of the Royal Society of London. Volume 176, 1885, pp. 691-723, doi: 10.1098 / rstl.1885.0014 .
- ^ Paul Émile Lecoq de Boisbaudran: Recherches sur le samarium. In: Comptes rendus . Volume 114, 1892, pp. 575-577 ( digitized on Gallica ).
- ↑ Eugène-Anatole Demarçay: Sur un nouvel élément contenu, dans les terres rares voisines du samarium. In: Comptes rendus. Volume 122, 1896, pp. 728-730 ( digitized on Gallica ).
- ^ Eugène-Anatole Demarçay: Sur un nouvel élément, europium. In: Comptes rendus. Volume 132, 1901, pp. 1484-1486 ( digitized on Gallica ).
- ^ William Crookes: On the Phosphorescent Spectra of Sδ and Europium. In: Proceedings of the Royal Society of London. Volume 76, No. 511, 1905, pp. 411-414 ( abstract ).
- ^ GT Seaborg, RA James, LO Morgan: The New Element Americium (Atomic Number 95). In: NNES PPR. (National Nuclear Energy Series, Plutonium Project Record). Vol. 14 B The Transuranium Elements: Research Papers. Paper No. 22.1, McGraw-Hill Book Co., New York 1949; Abstract ; Machine script (January 1948) (PDF; 1.9 MB).
- ^ Albert K. Levine, Frank C. Palilla: A new, highly efficient red-emitting cathodoluminiscent phosphor (YVO 4 : Eu) for color television. In: Applied Physics Letters. Volume 5, 1964, p. 118, doi: 10.1063 / 1.1723611 .
- ↑ Stephen B. Castor: Rare Earth Deposits of North America. In: Resource Geology. Volume 58, 2008, pp. 337-347, doi: 10.1111 / j.1751-3928.2008.00068.x .
- ↑ Harald Elsner: Critical supply situation with heavy rare earths - development of “green technologies” endangered? In: Commodity Top News. 2011, No. 36 (PDF)
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea, pp. 14-18.
- ↑ a b c d Ian McGill: Rear Earth Elements. In: Ullmann's Encyclopedia of Industrial Chemistry . Wiley-VCH, Weinheim 2012, doi: 10.1002 / 14356007.a22_607 .
- ↑ Gordon B. Haxel, James B. Hedrick, Greta J. Orris: Rare Earth Elements — Critical Resources for High Technology . United States Geological Survey Fact Sheet 087-02, 2002.
- ↑ Shyama P. Sinha: Systematics and the properties of the lanthanides. Springer, 1983, ISBN 90-277-1613-7 , pp. 550–551 ( limited preview in Google book search).
- ↑ DF Weill, MJ Drake: Europium Anomaly in Plagioclase Feldspar: Experimental Results and Semiquantitative Model. In: Science. Volume 180, 1973, pp. 1059-1060, doi: 10.1126 / science.180.4090.1059 .
- ^ Myron G. Best: Igneous and Metamorphic Petrology. Freeman, New York, 1982, ISBN 0-7167-1335-7 , p. 56.
- ^ SR Taylor, P. Jakes: The geochemical evolution of the moon. In: Lunar Science Conference, 5th, Houston, Tex., March 18-22, 1974, Proceedings. Volume 2, 1974, pp. 1287–1305 (full text)
- ^ SR Taylor: Lunar Science: A post-Apollo View. Pergamon, New York 1975, p. 156.
- ^ Steven Chu : Critical Materials Strategy. DIANE Publishing, 2011, ISBN 978-1-4379-4418-1 , pp. 87-88 ( limited preview in Google book search).
- ^ Abigail Walters, Paul Lusty: Rear Earth Elements. British Geological Survey , 2011 ( pdf, 4.7 MB ).
- ^ A b c d A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , pp. 1938-1944.
- ↑ CS Barrett: Crystal Structure of Barium and Europium at 293, 78, and 5 ° K. In: The Journal of Chemical Physics. Volume 25, 1956, p. 1123, doi: 10.1063 / 1.1743161 .
- ↑ K. Takemura, K. Syassen: Pressure-volume relations and polymorphism of europium and ytterbium to 30 GPa. In: Journal of Physics F: Metal Physics. Volume 15, 1985, pp. 543-559, doi: 10.1088 / 0305-4608 / 15/3/010 .
- ^ WA Grosshans, WB Holzapfel: X-ray studies on europium and ytterbium up to 40 GPa. In: Journal of Magnetism and Magnetic Materials. Volume 47-48, 1985, pp. 295-296, doi: 10.1016 / 0304-8853 (85) 90420-2 .
- ↑ M. Debessai, T. Matsuoka, J. Hamlin, J. Schilling, K. Shimizu: Pressure-Induced Superconducting State of Europium Metal at Low Temperatures. In: Physical Review Letters. Volume 102, 2009, pp. 197002-197005, doi: 10.1103 / PhysRevLett.102.197002 .
- ^ A b F. WD Rost: Fluorescence Microscopy. Volume 2, Cambridge University Press, 1995, ISBN 0-521-41088-6 , p. 291 ( limited preview in Google book search).
- ^ A b Peter Bamfield: Chromic phenomena. Technological applications of color chemistry. Royal Society of Chemistry, 2001, ISBN 0-85404-474-4 , p. 159 ( limited preview in Google book search).
- ↑ Arunachalam Lakshmanan: Luminescence and Display Phosphors. Phenomena and Applications. Nova Publishers, 2008, ISBN 978-1-60456-018-3 , p. 269 ( limited preview in Google book search).
- ^ A b John Emsley: Nature's building blocks. An A – Z guide to the elements. Oxford University Press, 2001, ISBN 0-19-850341-5 , pp. 139-141 ( limited preview in Google Book Search).
- ↑ P. Belli, R. Bernabei, F. Cappella, R. Cerulli, CJ Dai, FA Danevich, A. d Angelo, A. Incicchitti, VV Kobychev, SS Nagorny, S. Nisi, F. Nozzoli, D. Prosperi, VI Tretyak, SS Yurchenko: Search for α decay of natural Europium. In: Nuclear Physics. Volume A 789, 2007, pp. 15-29, doi: 10.1016 / j.nuclphysa.2007.03.001 .
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties. In: Nuclear Physics. Volume A 729, 2003, pp. 3-128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ^ Argonne National Laboratory : Europium ( Memento from December 16, 2011 in the Internet Archive ) (PDF; 93 kB). Human Health Fact Sheet, August 2005.
- ↑ Ralph F. Palumbo, Frank G. Lowman: The occurence of antimony-125, europium-155, iron-55, and other radionuclides in rongelap atoll soil. United States Atomic Energy Commission . 1958 (pdf)
- ^ Regino Saez, Paul A. Caro: Rare Earths. Editorial Complutense, 1998, ISBN 84-89784-33-7 , pp. 323–326 ( limited preview in Google book search).
- ↑ Pekka Hänninen, Harri Härmä: Lanthanide Luminescence. Photophysical, Analytical and Biological Aspects. Springer, 2011, ISBN 978-3-642-21022-8 , p. 220 ( limited preview in Google book search).
- ↑ Per Enghag: Encyclopedia of the Elements. John Wiley & Sons, 2008, ISBN 978-3-527-61234-5 , pp. 485-486 ( limited preview in Google Book Search).
- ↑ EP Klochkov, VD Risovanyi, Yu. E. Vaneev, AN Dorofeev: Radiation Characteristics of Europium-Containing Control Rods in a SM-2 Reactor after Long-Term Operation. In: Atomic Energy. Volume 93, No. 2, 2002, pp. 656-660, doi: 10.1023 / A: 1021096715382 .
- ↑ Simon Cotton: Lanthanide and Actinide Chemistry. John Wiley & Sons, 2007, ISBN 978-0-470-01007-5 , p. 77.
- ↑ Thomas J. Haley, N. Komesu, G. Colvin, L. Koste, HC Upham: Pharmacology and toxicology of europium chloride. In: Journal of Pharmaceutical Sciences . Volume 54, 1965, pp. 643-645, doi: 10.1002 / jps.2600540435 .
- ↑ Chitta Ranjan Patra, Soha S. Abdel Moneim, Enfeng Wang, Shamit Dutta, Sujata Patra, Michal Eshed, Priyabrata Mukherjee, Aharon thoughts, Vijay H. Shah, Debabrata Mukhopadhyay: In vivo toxicity studies of europium hydroxide nanorods in mice. In: Toxicology and Applied Pharmacology . Volume 240, 2009, pp. 88-98, doi: 10.1016 / j.taap.2009.07.009 .
- ↑ CR Patra, R. Bhattacharya, S. Patra, NE Vlahakis, A. Gabashvili, Y. Koltypin, A. Thought, P. Mukherjee, D. Mukhopadhyay: Pro-angiogenic Properties of Europium (III) Hydroxide Nanorods. In: Advanced Materials . Volume 20, 2008, pp. 753-756, doi: 10.1002 / adma.200701611 .
- ↑ RD Shannon: Revised effective ionic radii and systematic studies of interatomic distances in halides and chalcogenides. In: Acta Crystallographica Section A. 32, 1976, pp. 751-767, doi: 10.1107 / S0567739476001551 .
- ↑ Mihail Nazarov, Do Young Noh: New Generation of Europium- And Terbium-Activated Phosphors. From Syntheses to Applications. CRC Press, 2011, ISBN 978-981-4310-77-2 , pp. 280–282 ( limited preview in Google book search).
- ↑ Entry on europium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on March 27, 2012.
- ↑ H. Baernighausen, G. Brauer: A new europium oxide Eu 3 O 4 and the isotype compound Eu 2 SrO 4 . In: Acta Crystallographica. Volume 15, 1962, pp. 1059-1059, doi: 10.1107 / S0365110X62002807 .
- ↑ K. Binder: Spin glasses: Experimental facts, theoretical concepts, and open questions. In: Reviews of Modern Physics. 58, 1986, pp. 801-976, doi: 10.1103 / RevModPhys.58.801 .
- ↑ Switlana Manastyrskyj, Michael Dubeck: The Tetrahydrofuranate of europium (III) cyclopentadienides. In: Inorganic Chemistry. Volume 3, 1964, pp. 1647-1648, doi: 10.1021 / ic50021a044 .
- ^ EO Fischer, Hartmut Fischer: Europiumdicyclopentadienyl. In: Angewandte Chemie. Volume 76, 1964, pp. 52-52, doi: 10.1002 / anie.19640760114 .
- ^ William J. Evans, Laura A. Hughes, Timothy P. Hanusa: Synthesis and x-ray crystal structure of bis (pentamethylcyclopentadienyl) complexes of samarium and europium: (C 5 Me 5 ) 2 Sm and (C 5 Me 5 ) 2 Eu. In: Organometallics. Volume 5, 1986, pp. 1285-1291, doi: 10.1021 / om00138a001 .
- ↑ Christoph Elschenbroich : Organometallchemie. 6th edition. Teubner, Wiesbaden 2008, ISBN 978-3-8351-0167-8 , p. 577.