selenium
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Selenium, Se, 34 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Semi-metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 16 , 4 , p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | gray, shiny | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7782-49-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-957-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.029.052 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 0.8 ppm frequency # 59 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 78,971 (8) u | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 115 (103) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 120 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 190 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Ar ] 3 d 10 4 s 2 4 p 4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 9.752 392 (15) eV ≈ 940.96 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 21st.196 (10) eV ≈ 2 045.1 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 31.697 (19) eV ≈ 3 058.3 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 42.947 (3) eV ≈ 4 143.8 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 68.30 (10) eV ≈ 6 589.9 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 6. Ionization energy | 81.83 (3) eV ≈ 7 895 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 4.28 g / cm 3 (black Se at 60 ° C); 4.819 g / cm 3 (gray Se at 25 ° C); |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | diamagnetic ( Χ m = −1.9 10 −5 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 494 K (221 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 958.2 K (685 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 16.42 · 10 −6 m 3 · mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 95.5 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 5.4 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 3350 m s −1 at 293.15 K. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 5.9 eV | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 0.52 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | ± 2, 4 , 6 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −0.67 V (Se + 2 e - → Se 2− ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 2.55 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Selenium [ zeˈleːn ] is a chemical element with the element symbol Se and the atomic number 34. In the periodic table it is in the 4th period as well as the 6th main group or the 16th IUPAC group , so it belongs to the chalcogens . It comes in several modifications , the most stable being the gray, metal-like form.
history

Selenium ( Greek σελήνη [selḗnē], "moon") was discovered in 1817 by Jöns Jakob Berzelius in the lead chamber sludge of a sulfuric acid factory ; Berzelius first thought the substance was tellurium (from Latin tellus 'earth' ), to which selenium has some similarities; Thus, when both elements are burned, a distinct odor of radish develops. In 1818, however, Berzelius concluded in the course of his experiments that it was a new element; to indicate the similarity to tellurium (earth), he called it selenium (moon).
Occurrence
In inanimate nature and in industrial processes, the inorganic compounds play a role. Organic compounds dominate in living nature. In yeast and plants, selenium occurs primarily as selenomethionine . As an essential trace element, selenium is part of the 21st biogenic amino acid selenocysteine , as well as contained in bacteria, archaea and eukaryotes . Animals do not produce selenomethionine, but they do produce selenocysteine. Selenocysteine is the specific catalytic component of the selenium-dependent enzymes . In contrast to this, selenomethionine, instead of methionine, is incorporated non-specifically into many proteins without having any function; it is viewed as a selenium storage form. The amount of selenium in food is highly dependent on the selenium content of the soil. Soils poor in selenium in Europe are found particularly in Germany, Scotland, Denmark, Finland, in parts of the Balkan countries and in Switzerland. In some selenium-poor areas, fertilizers containing selenate are added to the soil, e.g. B. in Finland since 1984.
True selenium occurs naturally in small amounts . Selenium minerals such as clausthalite and naumannite are rare.
Selenium, usually in the form of metal selenides , accompanies sulfur-containing ores of the metals copper , lead , zinc , gold and iron . When roasting of ores , the solid collected selenium dioxide in the fly ash or in the downstream production of sulfuric acid as selenous acid .
Selenium can be enriched in tragacanth species, in Brassica or in garlic as Se- methylselenocysteine . The richest known source of selenium among foods is the Brazil nut .
Extraction and presentation
In industry, selenium is obtained as a by-product in the electrolytic production of copper and nickel from the anode sludge by roasting.
The reduction to elemental selenium takes place through sulfur dioxide :
On a laboratory scale, selenium can be produced through the reaction of selenous acid with hydrogen iodide .
Organically bound selenium
In food supplements and animal nutrition (approved for animal nutrition in the EU since May 2005), an organic selenium source has been used for several years, which is obtained by breeding certain brewing yeasts of the Saccharomyces cerevisiae type (Sel-Plex, Lalmin (TM)) on a nutrient medium rich in selenium ( Molasses and sodium selenite ) is produced. Yeasts synthesize high levels of selenomethionine as an amino acid and thus bind up to 2000 ppm selenium in an organic way. The largest plant for the production of such natural selenium yeasts was built in 2004 in São Pedro in the Brazilian state of Paraná .
properties
Like sulfur, selenium occurs in several modifications :
- Red selenium, soluble in carbon disulfide , consists of around 30% Se 8 rings and 70% Se 8 + n , which converts into the gray semiconductor metal above 80 ° C. Elemental red selenium is an insulator.
- Black amorphous selenium, which converts into black, vitreous selenium above 60 ° C. Both forms convert into the gray, semi-metallic modification when heated above 80 ° C.
- Gray “metallic” selenium is the most stable modification and behaves like a semi-metal.
- Above the melting point of 220 ° C it forms a black liquid. The selenium vapor produced when the temperature rises further is yellow.
- When it is deposited from the vapor phase on a cooler surface (a little below the melting point) it is deposited in the form of hexagonal, metallic-gray crystal needles.
The band gap of selenium is about 1.74 eV (on the border between visible light and infrared).
Exposure to light changes its electrical conductivity. In addition, it shows a photovoltaic effect . The conductivity is not caused by electrons in a conduction band , but by the conduction of holes (see under Electrical conductivity and defect electron ), i.e. positively charged electron defects, which among other things makes the sign of the Hall effect negative. A so-called “ hopping conductivity ” (of the holes from one crystal defect to the next) is proposed as a mechanism for this hole conduction .
When heated in air, selenium burns with a blue flame to form selenium dioxide , SeO 2 . Above 400 ° C it reacts with hydrogen to form hydrogen selenide , H 2 Se. It usually forms selenides with metals, e.g. sodium selenide , Na 2 Se.
The chemical behavior is similar to sulfur, but selenium is more difficult to oxidize. The reaction with nitric acid "only" forms selenium acid , a selenium (IV) compound.
Isotopes
Selenium has a multitude of isotopes . Of the six naturally occurring isotopes, five are stable. The proportions are distributed as follows: 74 Se (0.9%), 76 Se (9.0%), 77 Se (7.6%), 78 Se (23.6%), 80 Se (49.7% ) and 82 Se (9.2%).
82 Se, the only naturally occurring radioactive isotope, has one of the longest currently known half-lives of around 10-20 years . In addition, another 22 radioactive isotopes are known, of which 75 Se with a half-life of 120 days and 79 Se with a half-life of 327,000 years are of particular importance. 75 Se is used to construct special gamma radiation sources for non-destructive testing of e.g. B. Welds application. 75 Se is used in nuclear medicine in conjunction with methionine as a tracer to assess pancreatic function and with homotaurocholic acid (SeHCAT) to assess the absorption of bile acids . 79 Se is a component of spent nuclear fuel, where it is produced when uranium is fissioned with a frequency of 0.04%.
The rarest of the stable isotopes 74 Se has acquired a certain importance as an object of speculation. It is always offered on the market at very high prices. Except for some very specialized research applications where it is used for marking purposes, however, no particular technical use is known for this material.
use
Selenium is essential for all forms of life. Selenium compounds are therefore offered as food supplements and processed into feed and fertilizer additives. In the glass industry it is used to decolorize green glasses and to manufacture red-colored glasses. Other uses:
- Imaging drums for photocopiers and laser printers
- Semiconductor manufacturing
- Addition of latex to increase abrasion resistance
- Toner for black-and-white photographs to increase contrast (light tones remain unchanged, darker blacks can be achieved, the dark parts appear more three-dimensional overall), increase in durability (not clearly proven) and to lightly color the dark image components into aubergine-colored (also to increase plasticity )
- for the production of red color pigments based on cadmium selenide (due to the cadmium content rather rare today)
- Alloy additive to improve the mechanical workability of free- cutting steels and copper alloys
- Used in the selenium rectifier and the selenium cell , today however largely replaced by silicon ( semiconductor ).
- for browning aluminum, brass, etc. (Selenium dioxide)
- with copper and indium part of the photoactive layer of CIGS solar cells
- in analog light meters for photography
- Anti-dandruff hair shampoos and the prevention / therapy of pityriasis versicolor , a skin disease caused by a yeast fungus
- supportive in HIV therapy (disputed beneficial effect on HIV viral load)
- Reaction with Grignard compounds , R – Mg – Hal, leads to organoselenium compounds, R-Se-Mg-Hal, from which selenols , R – Se – H, can be produced by hydrolysis
- As zinc selenide , it is used to produce optically highly reflective surfaces, but it is transparent in the infrared range and is used here for the production of windows and focus lenses for e.g. B. CO 2 laser used
- Larger amounts of selenium dioxide are consumed in the electrolysis of manganese. The addition of selenium dioxide reduces the energy consumption during electrolysis. Up to 2 kg of selenium dioxide are used per ton of manganese.
Biological importance
Selenium is an essential trace element for humans, animals and many bacteria. Selenium is added to dairy cattle feeding because the natural selenium content of the feed is often insufficient to feed the livestock. German feed law only mentions the two inorganic selenium sources sodium selenite and selenate as feed additives to supplement the selenium supply . Both of these compounds are economically very favorable, but have the disadvantage of low bioavailability . In higher concentrations, however, selenium is highly toxic, with the range between concentrations that cause deficiency symptoms and toxic concentrations being very small. In addition, the toxicity of selenium depends on the chemical bond.
Selenium is contained in selenocysteine , an amino acid in the active center of the enzyme glutathione peroxidase and many other selenoproteins . Because of its high reactivity with oxygen, selenium plays an important role in protecting cell membranes from oxidative destruction ( radical scavenger ) in animals and humans . The selenium-containing enzyme glutathione peroxidase, which occurs in all animal cells, plays a decisive role in the breakdown of membrane-damaging oxidants and radical products. A number of selenium deficiency syndromes can be explained by reduced glutathione peroxidase activity. Such a connection is being discussed for cardiovascular diseases. The experimental hypertension in rats can also be significantly reduced by prophylactic administration of selenium. The protective selenium effect in the cryopreservation of heart muscle fragments is interesting in this context. Based on the saturation of selenoprotein P with selenium, the recommended daily dose in Germany, Austria and Switzerland was set at 70 μg per day for a man, 60 μg per day for a woman and 75 μg for women who are breastfeeding.
Selenocysteine is also involved in the catalytic mechanism of other enzymes and is contained in many proteins, the meaning of which has not yet been clarified.
Discussion about selenium
Before a working group led by Klaus Schwarz at the National Institute of Health (USA) discovered selenium as an essential food component of animals, selenium was considered a toxic substance. In the 1930s, veterinarians in the “ Great Plains ” blamed the high intake of selenium-containing plants for alkali disease and blind ataxia in cattle. On the other hand, a research group led by Schwarz reported in the 1950s that selenium prevents necrotic liver degeneration. Around the same time, a group of researchers from Oregon State University , including OH Muth and JE Oldfield, found a selenium deficit in weak calves. Hogue later demonstrated that selenium prevents muscular dystrophy in lambs. Following these reports, researchers from various institutions have begun studies on the benefits of selenium supplementation on dairy cow performance and health. It has been described that selenium is primarily involved in the catalysis of the glutathione peroxidase (GSH-Px) system. Different isoforms of GSH-Px destroy the peroxides (reactive oxygen compounds) formed during normal lipid metabolism. If peroxides remain unhindered in the cell, they attack the cell membranes and destabilize them. Hemken explained that selenium is also involved in detoxifying dangerous drugs or toxins. In animals, selenium also plays a role in at least two other enzyme systems: iodothyronine deiodase, an enzyme that activates the thyroid hormone T4 , and thioredoxin reductase, an enzyme that regulates the reducing reactions. Certain plasma, heart, muscle, and kidney proteins contain selenium. However, the function of selenium in these proteins is still largely unclear.
There are many different selenoproteins. Selenoproteins usually contain selenocysteine , which is also known as the 21st amino acid and which is incorporated via its own tRNA during protein synthesis. Selenoproteins only occur in this function in animal organisms, bacteria and archaea. Depending on the soil content, plants incorporate selenium unspecifically in amino acids instead of sulfur, especially in methionine (Se-methionine) and, to a lesser extent, cysteine (Se-cysteine) or derivatives thereof (methyl-Se-cysteine). Only the so-called “selenium collector plants” (selenium accumulator plants, e.g. paradise nut ), which occur in selenium-rich, arid areas, also store selenium as organically bound, water-soluble selenium or selenium salts.
To date, at least 25 genes for selenoproteins have been discovered in the human genome:
- Glutathione peroxidase 1 (GSHPx-1), the cellular or classic glutathione peroxidase (in the cytosol, mitochondrial matrix);
- Glutathione peroxidase 2 (GSHPx-2), the gastrointestinal glutathione peroxidase (in the intestinal mucosa);
- Glutathione peroxidase 3 (GSHPx-3), the extracellular or plasma glutathione peroxidase (in plasma);
- Glutathione peroxidase 4 (GSHPx-4), the phospholipid hydroperoxide glutathione peroxidase (on lipid membranes, structural protein in the tail of sperm); → antioxidant enzymes that neutralize peroxide radicals
- Thioredoxin reductase (TrxR) → reduces thioredoxin, which is important for cell growth, but also numerous other low-molecular and high-molecular substrates.
- Iodothyronine 5'-deiodinases (thyroid hormone iodinases) (ID-I, ID-II, ID-III) → catalyze thyroid hormones, for example removal of an iodine atom from T4 ( thyroxine ), whereby T3 ( triiodothyronine ) is formed
- Selenoprotein P (Se-P) → very important as a transport protein for selenium from and to the cells; contains 10 selenium atoms
- Selenoprotein W → in the muscles; Role still unknown
- Selenium phosphate synthetase → catalyzes the synthesis of monoselenophosphate, a precursor of selenocysteine
- H. Selenoprotein, M, N, O, I, K, S, V → The function of these selenoproteins is still largely not understood. Mutations in the SEPN1 gene have been described in multicore myopathy .
- Selenoprotein R = methionine sulfoxide reductase
- Selenophosphatase Synthetase 2 → catalyzes the production of selenophosphate
Selenium deficiency diseases
Known selenium deficiency diseases are:
- Keshan Disease (juvenile cardiomyopathy), named after the northeastern Chinese city of Keshan in the district of Heilongjiang in Manchuria
- Selenium deficiency promotes a mutation of the harmless Coxsackievirus B3 (CVB3 / 0), which becomes virulent as a result
- Occurrence: Tibet , Mongolia , Siberia
- Kashin-Beck disease in humans (nutritive articular cartilage degeneration), named after the Russian doctor Nikolai Iwanowitsch Kashin and the American Melinda A. Beck
- Occurrence: Siberia, Mongolia, North Korea, China; around 3 million people are affected
- Epidemic neuropathy in humans
- Occurrence: Cuba
- Selenium deficiency causes a mutation of the influenza A / Bangkok / 1/79 virus, which becomes virulent as a result
- White muscle disease (nutritive myodegeneration (NMD), nutritive muscular dystrophy , enzootic myodystrophy, nutritive rhabdomyolysis , nutritive rhabdomyopathy , myopathic - dyspnoic syndrome, calf rheumatism, chicken meatiness, fish meatiness)
- Occurrence: in all selenium-deficient areas on earth
- Animal species: young from v. a. Ruminants: calves, lambs, kids, dromedary and llama foals
- Overuse myopathy of rumining cattle ( paralytic myoglobinuria , exercise rhabdomyolysis)
- Occurrence: in all selenium-deficient areas on earth
- Animal species: v. a. Cattle from eight months
Selenium as a dietary supplement
In a critical evaluation of the pharmaceutical information from June 2005, it was determined that the studies available to date could not provide any indications of any benefit from an additional dose of selenium. Although a positive influence on various types of cancer seems possible, on the other hand favoring other cancers not unlikely. The "SELECT" study ( " Sel enium and Vitamin E C ancer Prevention T rial") should in this regard provide information and be completed, 2013. However, this was canceled in October 2008 because it could be demonstrated during the study that there is no improved protective effect compared to the placebo and a benefit could be ruled out. In this study, an increased incidence of prostate cancer with the administration of vitamin E and an increased development of diabetes with the administration of selenium were found, but neither was statistically significant.
As part of the renewed evaluation of data from a study, Saverio Stranges from the University of Buffalo came to the conclusion that of the 600 patients who took selenium (200 µg daily) about ten percent had type 2 diabetes after almost eight years . In the placebo control group, it was only six percent. To date, no potential cause for the increased risk of diabetes has been found. High levels of selenium in the blood correlate with the risk of developing diabetes. Thus, the pharmaceutical information from February 2008 also comes to the conclusion: "A critical attitude towards poorly documented concepts, behind which of course there is a great financial interest, has once again been confirmed." However, the study situation is not clear in this regard. For example, the von Stranges study assumed methodological errors, such as the lack of a previous family history , which should have ruled out an increased familial prevalence of diabetes mellitus within the selenium group, and the fact that the test subjects examined were people with high levels of solar radiation and Were exposed to chemicals, which is why the results were difficult to transfer to “average” test persons. In addition, the diabetes risk in both the placebo and selenium groups is below the American average. Other studies also suggest an inhibitory effect of selenium on the development of diabetes mellitus, including a recently published study by Tasnime Akbaraly (University of Montpellier) on 1,162 men and women.
A study from 2012 also shows a positive effect of selenium only when there is a selenium deficiency, otherwise diabetes mellitus is more likely to develop. A large meta-study from 2013 shows no protective benefits of selenium substitution with regard to cardiovascular diseases. Although there were increased diabetes 2 cases in the selenium substitution group, the difference was not significant. But there was more alopecia and dermatitis .
Sodium selenite and thyroid hormones
Selenium plays an important role in the production of thyroid hormones, more precisely in the "activation" of thyroxine (T4) to triiodothyronine (T3).
Selenium is part of an enzyme, thyroxine 5′-deiodase, which is responsible for removing an iodine atom from T4. This deiodization creates T3. A selenium deficiency leads to a deficiency in thyroxine 5′-deiodase, which means that only part of the available T4 can be deiodinated. Since T3 is much more effective in metabolism, a T3 deficiency results in an underactive thyroid ( hypothyroidism ). An additional intake of selenium preparations (sodium selenite) in high doses of 200-300 μg daily e.g. B. in Hashimoto's thyroiditis to reduce the inflammatory activity is discussed sporadically.
proof
The quantitative determination of traces (0.003%) of selenate can be carried out electrochemically by means of polarography . In 0.1 molar ammonium chloride solution , there is a step at −1.50 V (versus SCE ). In the ultra-trace range, atomic spectrometry can be used , whereby 100 μg / l ( ppb ) can be detected using flame AAS, 0.5 using graphite tube AAS and 0.01 μg / l using hydride technology.
safety instructions
Selenium and selenium compounds are poisonous. Direct contact damages the skin (blistering) and mucous membranes. Inhaled selenium can lead to protracted lung problems.
Poisoning from excessive consumption of selenium is known as selenosis . A selenium intake of more than 3000 µg / day can lead to liver cirrhosis , hair loss and heart failure . Employees in the electronics, glass and paint industries are considered to be at risk. According to other sources, symptoms of poisoning such as nausea and vomiting, hair loss, nail changes, peripheral neuropathy and exhaustion occur from 400 µg / d .
Selenium compounds
In compounds, selenium occurs most frequently in the oxidation states −II ( hydrogen selenide , selenides ) and + IV (tetrahalides, selenium dioxide and selenates (IV) , selenites out of date ). In the selenide ions, selenium also occurs with non-integer negative oxidation numbers. Less common positive oxidation numbers are + I (halides Se 2 X 2 ) and + VI ( selenium hexafluoride , selenic acid ). Selenium compounds with an oxidation number of + VI are stronger oxidizing agents than the analogous sulfur and tellurium compounds. Mixtures of concentrated selenium (VI) acid with hydrochloric acid dissolve metals such as gold and platinum.
Hydrogen compounds
Hydrogen selenide , H 2 Se, is a colorless, very poisonous gas that is produced by the reaction of selenides (M x Se y ) with strong acids, for example hydrochloric acid HCl. From the elements (hydrogen and selenium), the compound can only be represented as a strongly endothermic compound at temperatures above 350 ° C. Hydrogen selenide slowly decomposes into the elements at room temperature, the decomposition is accelerated by the influence of light. The aqueous solution ( hydro selenic acid) is weakly acidic; the acid strength (K s = 1.88 · 10 −4 ) is in the same order of magnitude as that of HNO 2 .
Selenides
With most metals, selenium forms binary selenides that contain the selenide anion Se 2− . In addition, Diselenide Se 2 2− and Polyselenide Se n m− are known, which can be obtained by the reaction of a metal with an excess of selenium:
The synthesis is possible by melting the elements together or in solution. The selenides are sensitive to hydrolysis and oxidation. Besides the ionic selenides, the molecular compound carbon diselenide , Se = C = Se, is known.
- Examples of polyselenides
Oxygen compounds and interchalcogens
Selenium dioxide ( selenium (IV) oxide ) is a colorless, crystalline solid that can be obtained by burning selenium in air. In water it forms selenium acid , H 2 SeO 3 . It is a relatively powerful oxidizer and is easily reduced to selenium.
Selenium trioxide ( selenium (VI) oxide ) can be obtained by dehydrating selenic acid , H 2 SeO 4 . It is also a crystalline solid and a strong oxidizing agent.
There are also the solid, crystalline, mixed-valent selenium (IV, VI) oxides Se 2 O 5 and Se 3 O 7 .
Selenium monoxide, SeO, is only known as an unstable intermediate.
Selenium sulfide SeS ≈2 (an unstoichiometric selenium-sulfur compound, which consists of sulfur-like cyclic molecules of variable size and composition, also called selenium disulfide because of the approximate ratio SeS 2 ).
Selenates are the salts of selenic acid with the anions SeO 4 2− . Orthoselenates such as the trigonal-bipyramidal anion SeO 5 4− and the octahedral SeO 6 6− are rarely observed.
Selenium halides
Selenium hexafluoride can be represented by the reaction of selenium with elemental fluorine. Although it is more reactive than sulfur hexafluoride , it does not react with water under normal conditions.
The most important selenium halides are the tetrahalides, but a selenium tetraiodide could not be synthesized. The tetrahalides can be represented from the elements. They can react as Lewis bases with formation of: SeX 3 + as well as Lewis acids (formation of SeX 6 2− ). The dihalides and monohalides known with all halogens are unstable.
Organic selenium compounds
Organic selenium compounds mainly occur in oxidation states <II, II and IV. The organic selenium compounds essentially comprise the following groups of substances;
- Selane (organic selenides) RSeR, e.g. B. dimethyl selenide
- Diselane ( Diselenide ) RSeSeR
- Triselane (Triselenide) RSeSeSeR
- Selenols RSeH
- Selenenyle RSeX
- Selenium oxides R-Se (= O) -R
- Selenone R 2 SeO 2
- Selone R 2 C = Se, the selenium analogues of the ketones
Selenium polycations
By carefully oxidizing selenium, numerous selenium polycations Se n x + can be produced and crystallized with a suitable counterion. The counterion must be a weak Lewis base since the selenium polycations are relatively strong Lewis acids. Suitable oxidizing agents are often halides of the transition metals, which give the desired compound directly at temperatures of typically 200 ° C:
Often the crystallization is successful under the conditions of chemical transport , but sometimes anhydrous solvents such as tin (IV) chloride or silicon tetrabromide have to be used.
If the metal halide is not a suitable oxidizing agent, as is usually the case with halides of the main group elements, the corresponding tellurium tetrahalides can be used as oxidizing agents:
By varying the counterion and the reaction medium, a wide variety of polycations could be produced; Mixed selenium-tellurium polycations can also be accessed by appropriate selection of the reactants in the synthesis.
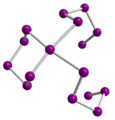
- Examples of selenium polycations
literature
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . Walter de Gruyter, Berlin, pp. 693-728.
- W. Marktl: Physiology and nutritional physiology of selenium. In: Journal for Mineral Metabolism . 8, No. 3, 2001, pp. 34-36 ( PDF ).
- PF Surai: Natural Antioxidants. Nottingham University Press, 2002, ISBN 1-897676-95-6 .
- Dolph L. Hatfield et al. (Ed.): Selenium: Its Molecular Biology and Role in Human Health. 3. Edition. Springer, New York 2012, ISBN 978-1-4614-1024-9 ( limited preview in Google Book Search).
- Gary S. Bañuelos et al. (Ed.): Selenium in the Environment and Human Health. CRC Press / Balkema, Leiden 2014, ISBN 978-1-138-00017-9 ( limited preview in Google book search).
Web links
- Office of Dietary Supplements Fact Sheet: Selenium
- Selenium in Cancer Medicine - Documentation ( Memento from February 28, 2013 in the Internet Archive ) (PDF file; 901 kB)
- Mineral Atlas: Selenium (Wiki)
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (selenium) , unless otherwise stated .
- ^ IUPAC, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e f Entry on selenium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e f Entry on selenium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics. CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics . Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b Entry on selenium in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ↑ Entry on Selenium in the Classification and Labeling Inventory of the European Chemicals Agency (ECHA), accessed on August 1, 2016. Manufacturers or distributors can expand the harmonized classification and labeling .
- ↑ Duden
- ↑ N. Figurowski: The discovery of the chemical elements and the origin of their names. 1981, p. 182.
- ↑ https://www.iupac.org/publications/ci/2011/3305/5_trofast.html
- ↑ Monitor selenium levels. In: schweizerbauer.ch . November 22, 2018. Retrieved November 22, 2018 .
- ^ Selenium in environmental medicine. In: Federal Health Gazette - Health Research - Health Protection. 49, 2006, p. 88, doi: 10.1007 / s00103-005-1185-4 .
- ↑ uni-duesseldorf.de: Ingredients Brazil nut . Retrieved May 29, 2013.
- ↑ E. Riedel, Christoph Janiak: Inorganic Chemistry . 8th edition. de Gruyter, 2011, ISBN 3-11-022566-2 , p. 458 .
- ^ R. Steudel: Chemistry of Non-Metals, 4th ed., De Gruyter, Berlin, 2013. ISBN 978-3-11-030797-9
- ^ NF Mott: Philosophical Magazine. 19, 835, 1969.
- ↑ http://www.webelements.com/selenium/isotopes.html
- ↑ ptb.de: The half-life of Se-79 .
- ↑ G. Jörg, R. Bühnemann, S. Hollas, N. Kivel, K. Kossert, S. Van Winckel, Ch. Lierse v. Gostomski: Applied Radiation and Isotopes. 68, 2010, pp. 2339-2351.
- ↑ Patent on gamma radiation source containing 75 Se .
- ↑ Torsten Kuwert, Frank Grünwald , Uwe Haberkorn , Thomas Krause (eds.): Nuclear medicine. Stuttgart 2008, ISBN 978-3-13-118504-4 .
- ↑ Selenium and Tellurium. ( Memento of November 13, 2011 in the Internet Archive ) (PDF; 51 kB) In: 2010 Minerals Yearbook. Retrieved May 29, 2013.
- ↑ Oehme, P. , W. Krause, E. Göres, N. Michael and O. Gomazkov: Selenium in medicine. In: Medicinal Therapy Heft 11, pp. 355-357 (1993).
- ↑ Krause, W. and P. Oehme : On the potential importance of selenium compounds for medicine. Part I: Selenium as an essential trace element - mechanisms of action, consequences of selenium deficiency. In: Dt. Gesundh.-Wesen 34, pp. 1713-1718 (1979).
- ↑ Krause, W. and P. Oehme : On the potential importance of selenium compounds for medicine. Part II: Some starting points for the therapeutic use of selenium compounds. In. German Gesundh.-Wesen 34, pp. 1769-1773 (1979).
- ↑ Hilse, H., P. Oehme , W. Krause and K. Hecht : Effect of sodium selenite on experimental hypertension in rat. In: Acta physiologica et pharmacologica bulgarica 1, No. 3. Pp. 47-50 (1979).
- ↑ Matthes, G., HA Hackensellner, KD Wagenbreth, H. Wendtlandt, P. Oehme and KD Jentzsch: Concentration dependency of the selenium effect in the context of the cryopreservation of heart muscle fragments. In: Probl. Häm. Transf. Transpl. 7, pp. 174-231 (1980).
- ^ AP Kipp, D. Strohm, R. Brigelius-Flohé, L. Schomburg, A. Bechthold, E. Leschik-Bonnet, H. Heseker: Revised reference values for selenium intake. In: Journal of trace elements in medicine and biology: organ of the Society for Minerals and Trace Elements. Volume 32, October 2015, pp. 195-199, doi : 10.1016 / j.jtemb.2015.07.005 , PMID 26302929 .
- ↑ KS Prabhu, XG Lei: Selenium. In: Advances in nutrition. Volume 7, number 2, March 2016, pp. 415-417, doi : 10.3945 / an.115.010785 , PMID 26980826 , PMC 4785479 (free full text).
- ↑ U. Schweizer, AU Bräuer, J. Köhrle, R. Nitsch, NE Savaskan: Selenium and brain function: a poorly recognized liaison. In: Brain research. Brain research reviews. Volume 45, Number 3, July 2004, pp. 164-178, doi: 10.1016 / j.brainresrev.2004.03.004 , PMID 15210302 (review).
- ↑ Are there any indications for selenium administration? In: Pharmainformation , Volume 20, No. 2, June 2005, accessed on May 29, 2013.
- ↑ Scott M. Lippman, Eric A. Klein et al. a .: Effect of Selenium and Vitamin E on Risk of Prostate Cancer and Other Cancers. In: JAMA . 301, 2009, p. 39, doi: 10.1001 / jama.2008.864 .
- Jump up ↑ S. Stranges, JR Marshall, R. Natarajan, RP Donahue, M. Trevisan, GF Combs, FP Cappuccio, A. Ceriello, ME Reid: Effects of Long-Term Selenium Supplementation on the Incidence of Type 2 Diabetes: A Randomized Trial . In: Annals of Internal Medicine . tape 147 , no. 4 , 2007, p. 217 .
- ↑ J. Bleys, A. Navas-Acien, E. Guallar: Serum Selenium and Diabetes in US Adults . In: Diabetes Care . tape 30 , no. 4 , 2007, p. 829-834 .
- ↑ Selenium - Diabetes. In: Pharmainformation , Volume 23, No. 1, February 2008.
- ↑ Critique of the study, published on Ask a laboratory doctor : Selenium and diabetes - hasty conclusions ( Memento of the original from March 14, 2012 in the Internet Archive ) Info: The archive link was inserted automatically and has not yet been checked. Please check the original and archive link according to the instructions and then remove this notice. .
- ↑ BioMed Central: Selenium Protects Men Against Diabetes, Study Suggests. In: ScienceDaily . March 18, 2010, July 28, 2010.
- ^ MP Rayman: Selenium and human health. In: The Lancet . 379 (9822), March 31, 2012, pp. 1256-1268, doi: 10.1016 / S0140-6736 (11) 61452-9 .
- ↑ K. Rees, L. Hartley, C. Day, N. Flowers, A. Clarke, S. Stranges: Selenium supplementation for the primary prevention of cardiovascular disease. In: The Cochrane Library. doi: 10.1002 / 14651858.CD009671.pub2 .
- ↑ D. Behne, A. Kyriakoupoulos, H. Meinhold, J. Koehrle: Identification of type I iodothyronine 5′-deiodinase as a selenoenzyme . In: Biochem. Biophys. Res. Comm. No. 173 , 1990, pp. 1143-1149 , PMID 2268318 .
- ^ JR Arthur, F. Nicol, GJ Beckett: Selenium deficiency, thyroid hormone metabolism, and thyroid hormone deiodinases . In: Am. J. Clinical Nutrition . No. 57 , 1993, pp. 236-239 ( abstract ).
- ↑ C. Ekmekcioglu: Trace elements on the way into the 21st century - increasing importance of iron, copper, selenium and zinc . In: Journal of Nutritional Medicine . No. 2 (2) , 2000, pp. 18–23 ( PDF edition for Austria).
- ↑ Influence of selenium substitution on the course of autoimmune thyroiditis .
- ↑ G. Schwedt: Analytical Chemistry . Thieme Verlag, Stuttgart 1995, p. 197 .
- ↑ Cornelia A. Schlieper: Selenium. In: Cornelia A. Schlieper: Basic questions of nutrition. Publishing house Dr. Felix Büchner, 2000, ISBN 3-582-04475-0 .
- ^ Robert M. Russell (for the German edition: Hans-Joachim F. Zunft): Vitamins and trace elements - deficiency and excess. In: Manfred Dietel, Joachim Dudenhausen, Norbert Suttorp (eds.): Harrison's internal medicine . Berlin 2003, ISBN 3-936072-10-8 .
- ^ J. Beck: Rings, cages and chains - The rich structural chemistry of the polycations of the chalcogens. In: Coordination Chemistry Reviews . 163, 1997, pp. 55-70, doi: 10.1016 / S0010-8545 (97) 00009-X .








![{\ displaystyle \ mathrm {17 \ Se \ + \ 2 \, WCl_ {6} \ longrightarrow \ Se_ {17} [WCl_ {6}] _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/9d84d24044480db3d71f417b3215a28035951a6e)
![{\ displaystyle \ mathrm {7 \ Se \ + \ SeCl_ {4} \ + \ 8 \ BiCl_ {3} \ longrightarrow \ Se_ {8} [Bi_ {4} Cl_ {14}] _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/3cb0faa868c47614d949e63102c492234888d14b)

