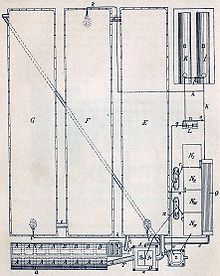
Lead chamber process

(A, B) Roasting ovens for pyrite ,
(C) saltpeter
oven with 2 bowls for sulfuric acid and nitric acid to release nitrous gases, (D) Gloverturm,
(E, F, G) lead chambers,
(H) Gay-Lussac tower.
The lead chamber process is a historic process for the production of sulfuric acid . This process was used on a larger scale in England at the beginning of the Industrial Revolution from 1746 in Birmingham , but had been known since the Middle Ages .
execution
To obtain sulfur trioxide, the sulfur was mixed with a little saltpetre as an additional oxidizing agent . The reaction product was then passed onto water. This was the original method of making sulfuric acid, but it was further developed for industrial production by John Roebuck in Birmingham in 1746 :
- Sulfur dioxide reacts with nitric acid to form sulfuric acid and nitrogen dioxide.
The glass bulbs were later replaced by large chambers lined with lead , the only metal that can withstand the very aggressive vapors and sulfuric acid that occur here, as it is covered with a protective layer of lead sulfate . The cumbersome addition of saltpetre was avoided when it was noticed that nitrogen dioxide also oxidizes the sulfur dioxide. It is reduced to monoxide itself and immediately reacts again with the oxygen in the air to form dioxide and repeats the process:
- Sulfur dioxide reacts with nitrogen dioxide to form sulfuric acid and nitrogen monoxide.
As a result, the sulfur dioxide is further oxidized by the oxygen in the air and the nitrous gases act as catalysts . The process was optimized through the introduction of the Glover and Gay-Lussac towers. This not only allowed an effective recycling of nitrogen oxides, but also a concentration of the sulfuric acid to approx. 80% ("gloversic acid"). The concentration of the “chamber acid”, on the other hand, was only 63… 70%. To obtain concentrated 96 percent sulfuric acid, the chamber acid was evaporated in large glass or platinum retorts.
Current applications in flue gas cleaning
The lead chamber process is used today in the flue gas cleaning of gases with relatively high SO 2 concentrations (> 0.5% by volume), such as occur, for example, in the roasting of molybdenum ores. As already mentioned above, the nitrogen oxides contained in the flue gas are used as a catalyst substance or possibly supplemented from a further nitrogen oxide source.
The process can be used in the future in connection with the purification of CO 2 separated from power plant processes . For easier final storage, the separated CO 2 is compressed in order to ultimately liquefy it. At a pressure of 30 bar, the lead chamber process can be carried out in a simplified manner in a single absorption scrubber with 70 wt% sulfuric acid, since the speed-limiting oxidation reaction of the nitrogen monoxide takes place at an accelerated rate. In this way, the CO 2 to be stored can be almost completely desulfurized.
References
- ^ H. Ost: Textbook of Technical Chemistry , published by Robert Oppenheim, Berlin, 1890, p. 53.
- ^ H. Ost: Textbook of Technical Chemistry , published by Robert Oppenheim, Berlin, 1890, p. 54.
- ^ H. Ost: Textbook of Technical Chemistry , published by Robert Oppenheim, Berlin, 1890, p. 55 f.
- ↑ U. Sander, V. Fattinger: Chemical Engineering and Economic Aspects of Converting Dilute SO 2 gas into Sulfuric Acid ,. Chem-Ing-Tech: 55 (8): 601-7 (1983)
- ↑ B. Keilin, 1972, Development of the catalytic chamber process for the manufacture of sulfuric acid and nitric acids from waste flue gases., Govt. Pep. Announce, vol. 72 (13)
- ↑ RJ Allam, V. White, EJ Miller: Air Products and Chemicals, Inc., (US), Purification of carbon dioxide, US2007 / 0122328A1 (2007)