Flue gas cleaning
The flue gas cleaning is used for removing pollutants from flue gas with the purpose of reducing environmental pollution. This process is a special case of exhaust gas cleaning .
How a flue gas cleaning system is constructed depends, among other things, on the area of application, the environment and the cleaning effort. Flue gas cleaning systems are used wherever the air must mainly be cleaned of solid particles ( dust ) before it gets into the atmosphere. This mainly applies to coal-fired power plants and waste incineration plants .
Which substances are being reduced?
In addition to non-hazardous components such as water vapor and nitrogen, smoke gases can contain the following pollutants:
- Carbon monoxide
- Sulfur dioxide
- Nitrogen oxides
- Hydrocarbons (also " VOC ")
- hydrochloric acid
- Hydrofluoric acid
- Heavy metals
- Dioxins and furans
- Fly ash
- soot
Some of these substances escape as dusty particles with different grain sizes or as aerosols (mixture of solid and / or liquid particles).
The cleaning consists essentially of filtering, adsorption and absorption and catalytic conversion. Some reaction products can be recycled, especially gypsum from desulphurisation plants in coal-fired power plants ( FGD gypsum ), and hydrochloric acid from flue gas scrubbers in waste incineration plants . Fly ash from coal-fired power stations has properties similar to cement and is used in high proportions as an aggregate in cement production.
The minimum requirements for flue gas cleaning are essentially defined in the Federal Immission Control Ordinance ( BImSchV ) and the technical instructions for keeping the air clean ( TA Luft ). In accordance with the EU directive on the integrated prevention and reduction of environmental pollution (" IVU directive " 96/13 / EC or 2008/1 / EC), the requirements for air pollution control must match the stipulations of the European "leaflets for best available technologies " (short "BVT information sheets") published by the EU Commission.
historical overview
At the beginning of the 20th century, despite increasing industrialization, flue gas cleaning was still neglected. Pollutant emissions and the associated air pollution were often not regulated by law. The technical and financial effort to reduce pollutants stood in the way of the steadily increasing production goals of the growing economy. It was not until the middle of the 20th century that air pollution began to be seriously addressed. Effective popular protests against this pollution began in the industrialized countries - North America, Europe and Japan - from the mid-1960s. These activities led to the establishment of the first state environmental protection institutions and to laws on air pollution control, for example the Federal Immission Control Act in Germany 1974.
However, only regional and local pollution was initially reduced by simply spreading it further through higher chimneys. As a result, sulfur and nitrogen oxides spread over thousands of kilometers and led to the formation of " acid rain ". For this reason, investments were also made in flue gas cleaning, as coal-fired power plants and waste incineration plants (MVAs) have long been among the largest sources of pollution. In 2001, a study commissioned by the environmental organization Greenpeace found that numerous pollutants from incinerators are still being released into the atmosphere. Because of this, and because of the ever stricter limit values, the flue gas cleaning systems are subject to constant development, research and control, with research being carried out in particular on the pollutant components dioxins and mercury .
Construction of a flue gas cleaning system
The structure of a flue gas cleaning system is divided into several different stages. Most flue gas cleaning systems for waste incineration plants consist of the five stages described below. Some of the cleaning stages described below are also used in coal-fired power plants. After the cleaning process, the excreted air gas mixtures are recorded at a sampling point and the measurement results are recorded before they then escape into the atmosphere via a chimney.
In the first stage, most of the dust is removed from the flue gas. This is done with a fabric filter or with an electrostatic precipitator .
Dedusting
Fabric filter
The task of the fabric filter is to separate dust particles from the smoke gases. The dust particles are mainly deposited on the outside of the filter material and form a so-called filter cake , which itself then acts as a highly efficient separator . Due to the filter cake, the differential pressure in the filter system and thus the energy consumption increase continuously, which means that regular regeneration is necessary (the regeneration intervals are between a few minutes and a few hours , depending on the dust concentration ). This is usually done by a compressed air pulse introduced against the direction of flow, which causes the filter cake to be thrown off. The discarded filter cake is usually removed from the collecting bunker of the filter system by means of a rotary valve .
In addition, acidic pollutant gases, dioxins / furans and heavy metals can also be separated in the fabric filter. This is done by adding additives such as lime or sodium hydrogen carbonate to separate acidic pollutant gases (through chemical reaction ) or activated carbon to separate dioxins / furans and heavy metals (through adsorption ) from the flue gas, which separates the pollutants in the entrained flow and in the fixed - bed reactor Filter cake is achieved. The amount of additives added can exceed the amount of dust particles from the combustion by a multiple (sometimes a factor of 100 or more). Since the additives are often not used up after a single use, only a certain proportion of the discarded filter cake is disposed of and the rest is added to the flue gas before the filter system (recirculation).
Electrostatic precipitator
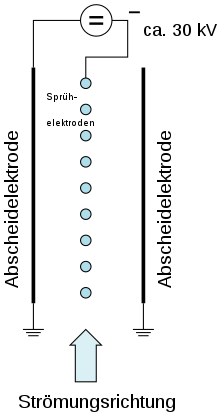
In some systems, the flue gas emerging from the boiler is first dedusted in the electrostatic precipitator. With electrical dust separation, dust particles are negatively charged in the gas flow with the aid of spray electrodes and deposited on opposite precipitation anodes. A direct voltage of 30 to 80 kV is applied between the spray and precipitation electrodes. The specific resistance of the charged dust is decisive for the separation. If this is too high, there is no longer any separation. The separated ash is transported to an ash silo. It is moistened with water either dry by tank wagon or in a mixing screw and then transported away.
Gas scrubbing
HCl absorber
To generate the purest possible hydrochloric acid , the hydrogen chloride absorber (HCl absorber) is usually designed in three stages. The first stage is used to cool the flue gases to saturation temperature, as well as the residual separation of dust and heavy metals. In a pipe cooling system made of temperature- and acid-resistant material, the hot flue gases are exposed to the circulating washing solution. The washing solution partially evaporates. Due to the strong turbulence of the flue gases at the outlet of the cooler and the low pH value (approx. 0) of the solution, dust and heavy metals are separated. Since there are no high demands on the quality of the cooling liquid, the wastewater from the other cleaning stages can be used as a washing solution. The rest can be filled with fresh water, where mostly roughly purified river water is sufficient. In this stage of the HCl absorber, gaseous mercury is particularly well separated. The second stage of the HCl absorber (HCl concentration stage) serves as a collector for the washing liquid, which flows from there into a container. The flue gases flow through a packed bed . The scrubbing liquid is distributed above the packing via channels and fed through the packing in countercurrent to the flue gases. The third stage (HCl fine cleaning stage) has the same structure as the concentration stage. In addition, a water separator prevents the formation of mist droplets. The chemical compounds are washed (converted) by the packing. The washing liquid is fed discontinuously through the second and third stages until a specified clean gas value is met.
- Advantages of a division into three separate levels
- The separation of the cooler from the concentration stage reduces the foreign matter content of the hydrochloric acid to be processed
- Much higher concentrations of crude hydrochloric acid are also made possible, which reduces the heating energy of the subsequent cleaning stages to 50%
- the HCl pure gas value is usually close to the detection limit, it only approaches the set value towards the end of the cycle
- For this reason, the HCl absorber offers an excellent buffer against sudden pollution peaks
SO 2 absorber
In the SO 2 scrubber is used in a wash cycle SO 2 deposited and reacted with hydrated lime to form gypsum. The consumption of hydrated lime is balanced out by adding milk of lime. To separate the gypsum that has formed, a partial flow of the washing cycle is discharged and fed to the gypsum preparation. Here the suspension is drained using a vacuum belt filter. The gypsum obtained is temporarily stored in the gypsum silo until it is removed. The washing water resulting from the drainage is fed back into the washing cycle. After exiting the SO 2 scrubber, the cleaned flue gas is heated to 105 ° C by means of a steam-heated heat exchanger and released into the atmosphere through the chimney with the help of the flue gas fan.
Dry method
Denitrification
Measures taken during the firing process can reduce nitrogen oxide levels by up to 30%. However, they are not always sufficient to meet the strict requirements for air pollution control. Therefore, further techniques had to be developed, which are called secondary measures. The two main technical processes are the SCR process and the SNCR process.
SCR process
In the SCR process , the Selective Catalytic Reduction , ammonia (NH 3 ) is injected into the flue gas flow; this causes the nitrogen oxides to convert into nitrogen (N 2 ) and water (H 2 O). This chemical reaction is accelerated by a catalyst. In order to prevent the formation of ammonium salts that would clog the pores of the catalyst, the catalysts are usually operated at temperatures of over 320 ° C. These salts do not form above this temperature. The catalyst can be placed in front of the air preheater (LUVO) and thus also in front of the electrostatic precipitator for dedusting. This is the so-called "high-dust" circuit. It has the advantage that the flue gases are already at the required temperature. However, in this case the flue gases are not yet dedusted, which can be disadvantageous for the catalytic converter. If the catalytic converter is arranged after the electrical or hose filter, the so-called "low-dust" circuit, the flue gas flow is already dedusted, but it may be necessary to reheat the already cooled flue gases.
SNCR procedure
The SNCR process , the Selective Non Catalytic Reduction , does not use a catalyst. Ammonia or urea is fed into the combustion chamber via nozzles. Here, too, the nitrogen oxides are converted into nitrogen and water. Depending on the load range in which the power plant is currently working, the location of the injection must be varied in order to ensure the optimal process temperature of 850 - 1000 ° C. This procedure requires a sophisticated regulation. NO x reductions of up to more than 80% are achieved, and an equally high dioxin and furan reduction can be achieved. However, the NO x reduction is usually below that of the SCR process, so that the SCR process must be used in the case of particularly strict limit values (the limit values prescribed in the approval notice for waste incineration plants are sometimes well below the legal limit values ).
Activated carbon filter
In this stage, residual organics still contained in the flue gas, such as halogenated hydrocarbons and dioxins as well as the last remnants of mercury and other heavy metals, are adsorbed by activated carbon . For this purpose, activated charcoal in the form of dust is metered into the flue gas flow and then deposited again together with the accumulated pollutants on the filter bags of the fabric filter. The used coal is discharged, packed in barrels and fed to the energetic recovery; it is often burned again in the ovens in the same power station.
Limit values and regulations (Germany)
| 1990 | 2008 | |
|---|---|---|
| Nitrogen oxides | 350 mg | 60 mg |
| Sulfur dioxide | 690 mg | 1.3 mg |
| cadmium | 175 µg | 1.4 µg |
| mercury | 12 µg | 0.1 µg |
| Dioxin equivalent | 10 ng | 0.01 ng |
17. Ordinance for the implementation of the Federal Immission Control Act (Ordinance on the incineration and co-incineration of waste - 17th BImSchV)
The amending ordinance amending the 17th BImSchV and the new version of the ordinance were announced in the Federal Law Gazette on August 19, 2003 ( Federal Law Gazette I p. 1614, 1633 ). The amended regulation came into force on August 20, 2003. A tightening came into force on January 31, 2009 ( Federal Law Gazette I, p. 129 ), which also stipulated a maximum annual mean nitrogen oxide concentration.
The amendment to the 17th BImSchV was used to implement the requirements of EU Directive 2000/76 / EC on the incineration of waste into national law. With it, the high level of immission control requirements for waste incineration plants already in force in Germany was laid down for all plants.
With the amendment of the 17th BImSchV, the requirements for co-incineration plants, such as power plants or cement works that use waste as substitute fuel, have been largely aligned with those of classic waste incineration plants ("mono-incineration"). To this end, new, demanding emission limit values have been set for co-incineration in particular , which replace the so-called “mixing rule” that was previously in force. In addition, the 17th BImSchV lays down requirements for the acceptance and storage of waste and incineration residues, for the measurement of emissions and for the use of waste heat.
| Pollutant | abbreviation | Limit value (pure gas) in mg / m³ |
|---|---|---|
| dust | 10 | |
| Hydrogen chloride | HCl | 10 |
| Sulfur dioxide | SO 2 | 50 |
| Hydrogen fluoride | HF | 1 |
| Carbon monoxide | CO | 50 |
| Nitrogen dioxide | NO 2 | 200 * |
| mercury | Ed | 0.05 |
| Cadmium + thallium | Cd, Tl | 0.05 |
| other heavy and semi-metals | (As, Cd, Co, Cr, Cu, Mn, Ni, Pb, Sb, Sn, V) | 0.5 |
| Sum of particularly toxic substances | (As, Cd, Co, Cr (VI), BaP) | 0.05 |
| Dioxins & Furans | 0.1 ng TE / m³ | |
| * 100 mg / m³ as an annual mean |
Comparison of electrostatic precipitators and fabric filters
This section compares the electrostatic precipitator and the bag filter . One of these two separators is used in every cleaning system to remove dust from the flue gas.
Electrostatic precipitator
- advantages
- robustness
- insensitive to temperature peaks
- Operation cheaper than bag filters
- disadvantage
- higher investment costs than fabric filters
- less good dust separation
- Electrostatic precipitators have wear parts that cannot be neglected (e.g. knocking mechanism, knocked plates, etc.)
Fabric and bag filters
- advantages
- better dust separation than electrostatic precipitators ( degree of separation well over 99%)
- By adding sorbents , gaseous pollutants can be separated in the filter cake
- lower investment costs
- disadvantage
- higher energy consumption / operating costs
- limited service life of the filter bags
- high temperature sensitivity
literature
- Karl J. Thomé-Kozmiensk (Ed.): Waste incineration and the environment. Volume 5. EF-Verlag für Energie- und Umwelttechnik, Berlin 1991, ISBN 3-924511-56-X .
- VDI reports 667: Flue gas cleaning-SO 2 / NO x . Ecological, economic and technical aspects. Hanover conference, February 24 and 25, 1988. VDI-Verlag, Düsseldorf 1988, ISBN 3-18-090667-7 .
- Helmut Rechberger : Thermal processes of disposal. Technical University of Vienna.