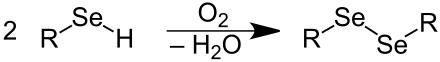Selenols
Selenols are carbon-containing (organic) selenium compounds , more precisely the selenium analogues of thiols (RSH) and alcohols (ROH). They have the general formula R-Se-H. The selenols can also be viewed as monosubstitution products of hydrogen selenide (H 2 Se). Selenocysteine is an amino acid that belongs to the selenol class.
synthesis
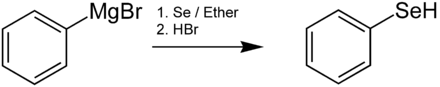
Alkyl selenols are mostly produced by reacting alkali hydrogen selenides with alkyl halides , by reducing diselenides or alkyl selenocyanates . The volatile alkyl selenols have a horrible odor and are poisonous. Selenophenol can be produced from organometallic compounds and elemental selenium , e.g. B. from Grignard compounds according to the following reaction sequence:
In the first reaction step, an insertion reaction creates a compound of the PhSeMgBr type. Selenophenol was first produced by the reaction of benzene with selenium tetrachloride (SeCl 4 ) in the presence of aluminum trichloride (AlCl 3 ).
Reactivity
Selenols, like thiols (mercaptans), form metal selenolates (selenomercaptides). The heavy metal selenolates are usually much more stable than the selenols and are therefore often used for further reactions. Sodium and potassium selenolates are formed when the selenols are heated with the alkali metal in benzene , toluene or xylene . Selenols are oxidized to diselenides by oxygen in the air :
If a selenol is reacted with a sodium alcoholate and then with a haloalkane , a dialkyl selenide, R-Se-R ' , is formed in a chemical reaction analogous to Williamson's ether synthesis .
Individual evidence
- ↑ a b Heinrich Rheinboldt in Houben-Weyl Methods of Organic Chemistry , edited by Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler, Volume 9, sulfur, selenium and tellurium compounds , Thieme Verlag, Stuttgart, 1955, there 952– 969
- ↑ Chabrié, M. Camille: Premiers essays de synthèse de composés organiques séléniés dans la série aromatique, Bull. Soc. Chim. Fr. , 50, 133 (1888); Ann. chim. phys., (6) 20, 229 (1890).