Selenium tetrachloride
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Se 4+ __ Cl - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Selenium tetrachloride | |||||||||||||||
| other names |
Selenium (IV) chloride |
|||||||||||||||
| Ratio formula | SeCl 4 | |||||||||||||||
| Brief description |
yellowish solid with a pungent odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 220.77 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.6 g cm −3 (25 ° C) |
|||||||||||||||
| Melting point |
305 ° C (under pressure) |
|||||||||||||||
| Sublimation point |
196 ° C |
|||||||||||||||
| solubility |
|
|||||||||||||||
| Refractive index |
1.807 (20 ° C) |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . Refractive index: Na-D line , 20 ° C | ||||||||||||||||
Selenium tetrachloride is an inorganic chemical compound of selenium from the group of chlorides .
Extraction and presentation
Selenium tetrachloride can be obtained by reacting selenium with chlorine .
properties
Selenium tetrachloride is a colorless to yellowish moisture-sensitive solid with a pungent odor, which decomposes with water and in moist air to form selenic acid and hydrochloric acid.
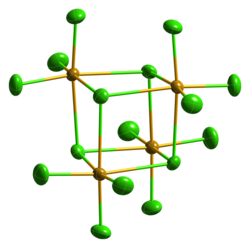
Selenium tetrachloride sublimes when heated. In the closed tube it melts at around 305 ° C to a dark red liquid. It crystallizes cubically in the space group P 4 3 n (space group no. 218) with the lattice parameter a = 16.433 Å . A metastable, monoclinic structure is also known (space group C 2 / c (No. 15) , a = 16.548, b = 9.81, c = 15.029 Å, β = 116.95 °). In concentrated hydrochloric acid, SeCl 4 forms hexachloroselenates (IV) with alkali chlorides, such as B. the yellow cesium hexachloroselenate (IV) Cs 2 [SeCl 6 ].
Individual evidence
- ↑ a b c d e data sheet Selenium (IV) chloride at AlfaAesar, accessed on November 24, 2013 ( PDF )(JavaScript required) .
- ↑ a b c d data sheet Selenium tetrachloride from Sigma-Aldrich , accessed on May 25, 2017 ( PDF ).
- ↑ a b c Georg Brauer , with the assistance of Marianne Baudler a . a. (Ed.): Handbook of Preparative Inorganic Chemistry . 3rd, revised edition. tape I . Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , pp. 417 .
- ^ Henry G. Nowak and John F. Suttle: Selenium (IV) chloride . In: Therald Moeller (Ed.): Inorganic Syntheses . tape 5 . McGraw-Hill, Inc., 1957, pp. 125-127 (English).
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 720 ( limited preview in Google Book Search).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 621.
- ^ Wilhelm Ostwald: Basic lines of inorganic chemistry . BoD - Books on Demand, 1922, ISBN 3-86195-686-1 , pp. 351 ( limited preview in Google Book Search).
- ^ R. Kniep, L. Korte, D. Mootz: Crystal structure of the stable modification of SeCl 4 . In: Zeitschrift für Naturforschung, Part B , 36, 1981, pp. 1660–1662, doi : 10.1515 / znb-1981-1231 .
- ↑ P. Born, D. Mootz, R. Kniep, M. Hein, B. Krebs: Phase relations in the system Se-SeCl 4 and the crystal structure of a metastable modification of SeCl 4 . In: Zeitschrift für Naturforschung, Part B , 36, 1981, pp. 1516-1519, doi : 10.1515 / znb-1981-1206 .
- ↑ Synthetic Methods of Organometallic and Inorganic Chemistry, Volume 4, 1997: Volume 4: Sulfur, Selenium and Tellurium, WA Herrmann and Christian Erich Zybill, Georg Thieme Verlag, 2014, ISBN 3131794410




