Selenium (I) chloride
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
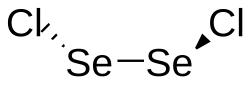
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Selenium (I) chloride | |||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | Se 2 Cl 2 | |||||||||||||||
| Brief description |
dark red liquid with a sour odor |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 228.83 g mol −1 | |||||||||||||||
| Physical state |
liquid |
|||||||||||||||
| density |
2.77 g cm −3 |
|||||||||||||||
| Melting point |
−85 ° C |
|||||||||||||||
| boiling point |
127 ° C (decomposition) |
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Selenium (I) chloride is an inorganic chemical compound of selenium from the group of chlorides .
Extraction and presentation
Selenium (I) chloride can be obtained by reacting a solution of selenium dioxide in hydrochloric acid with selenium.
It can also be represented by the reaction of selenium and sulfur trioxide with hydrogen chloride .
properties
Selenium (I) chloride is a dark red, slightly brownish, oily and moisture-sensitive liquid that is soluble in chloroform , benzene , carbon tetrachloride and carbon disulfide . It smells similar to disulfur dichloride and slowly hydrolyzes with water to form selenic acid , hydrochloric acid and selenium. Solid selenium (I) chloride has a monoclinic crystal structure with the space group P 2 1 / n (space group no. 14, position 2) .
See also
Individual evidence
- ↑ a b c d e f g h i j Data sheet Selenium (I) chloride, 99% from AlfaAesar, accessed on November 22, 2013 ( PDF )(JavaScript required) .
- ↑ a b Georg Brauer (Ed.), With the collaboration of Marianne Baudler u a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 416.
- ^ Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 720 ( limited preview in Google Book Search).




