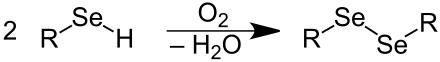Diselenide
Diselenides are covalent chemical compounds of the type R – Se – Se – R ′. If the organic radicals R and R 'are the same, it is a symmetrical diselenide. If the radicals R and R ′ are different, the diselenide is asymmetrical. Ionic (mostly inorganic) compounds that contain the diselenide ion - Se-Se - are also called diselenides, but represent a separate group of substances. Both groups of substances can be viewed as derivatives of the unstable diselane H 2 Se 2 . Diselenides are the selenium analogues of the disulfides .
synthesis
Symmetrical organic diselenides can be prepared by direct introduction of the diselen group into organic molecules or by oxidation of selenols . The autoxidation of selenols in air provides Diselenide:
Reactions
The reduction of organic diselenides produces selenols. Sodium is often used as a reducing agent .
Individual evidence
- ^ Heinrich Rheinboldt in Houben-Weyl Methods of Organic Chemistry , edited by Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler, Volume 9, Sulfur, Selenium and Tellurium compounds , Thieme Verlag, Stuttgart, 1955, there pages 1086-1105 .
- ^ Heinrich Rheinboldt in Houben-Weyl Methods of Organic Chemistry , edited by Eugen Müller, Otto Bayer, Hans Meerwein and Karl Ziegler, Volume 9, Sulfur, Selenium and Tellurium Compounds , Thieme Verlag, Stuttgart, 1955, there pages 955 and 961 .