Praseodymium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Praseodymium, Pr, 59 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Lanthanoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | La , 6 , f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | silvery white, yellowish hue |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-10-0 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-120-3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.291 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 5.2 ppm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 140.90766 (2) and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 185 (247) pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 203 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Xe ] 4 f 3 6 s 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 5.4702 (4) eV ≈ 527.79 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 10.631 (20) eV ≈ 1 025.7 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 21st.6237 (25) eV ≈ 2 086.37 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 38.981 (25) eV ≈ 3 761.1 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 57.53 (5) eV ≈ 5 550 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
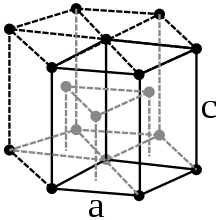
| Crystal structure | hexagonal | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 6.475 g / cm 3 (25 ° C ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 2.9 · 10 −3 ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1208 K (935 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 3403 K (3130 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 20.80 · 10 −6 m 3 · mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 331 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 6.9 kJ mol −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Speed of sound | 2280 m s −1 at 293.15 K. | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 1.43 · 10 6 A · V −1 · m −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 13 W m −1 K −1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | 3 , 4, 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −2.35 V (Pr 3+ + 3 e - → Pr) |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 1.13 ( Pauling scale ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NMR properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Praseodymium is a chemical element with the element symbol Pr and the atomic number 59. In the periodic table it is in the group of lanthanoids and is therefore also one of the rare earth metals . The name comes from the green color of its connections : the Greek word πράσινος prásinos means “green”, δίδυμος didymos “double” or “twin”.
history
In 1841 Carl Gustav Mosander extracted the rare earth didymium from lanthanum oxide . In 1874 Per Teodor Cleve noticed that Didym actually consisted of two elements. In 1879 Lecoq de Boisbaudran isolated samarium from Didym, which he extracted from the mineral samarskite . In 1885 Carl Auer von Welsbach succeeded in separating didymium into praseodymium and neodymium , which both form salts with different colors.
Occurrence
Praseodymium occurs naturally only in chemical compounds associated with other lanthanoids , mainly in the minerals
|
in front.
The global reserves are estimated at 4 million tons.
Extraction and manufacture
As with all lanthanides , the ores are first enriched by flotation , then the metals are converted into corresponding halides and separated by fractional crystallization , ion exchange or extraction .
The metal is obtained by fused-salt electrolysis or reduction with calcium .
properties
Physical Properties
Praseodymium is a soft, silver-white paramagnetic metal, which belongs to the lanthanides and rare earth metals . At 798 ° C, the hexagonal α-Pr converts to the body-centered cubic β-Pr.
Chemical properties
At high temperatures, praseodymium burns to sesquioxide Pr 2 O 3 . It is somewhat more corrosion-resistant in air than europium , lanthanum or cerium , but easily forms a green oxide layer that flakes off in air. It reacts with water to form praseodymium hydroxide (Pr (OH) 3 ) , forming hydrogen . Praseodymium is trivalent and tetravalent in its compounds, the trivalent oxidation number being the more common. Pr (III) ions are yellow-green, Pr (IV) ions are yellow. Bivalent praseodymium can also be produced under special reductive conditions, e.g. B. in praseodymium (II, III) iodide (Pr 2 I 5 ).
Isotopes
Natural praseodymium consists only of the stable isotope 141 Pr. 38 other radioactive isotopes are known, with 143 Pr and 142 Pr being the longest-lived with a half-life of 13.57 days and 19.12 hours, respectively. All other isotopes have half-lives of less than 6 hours, most even less than 33 seconds. There are also 6 metastable states, with 138 m Pr (t ½ 2.12 hours), 142 m Pr (t ½ 14.6 minutes) and 134 m Pr (t ½ 11 minutes) being the most stable.
The isotopes move in an atomic mass range from 120.955 ( 121 Pr) to 158.955 ( 159 Pr).
use
- Praseodymium is used in alloys with magnesium to make high-strength metal for aircraft engines.
- Alloys with cobalt and iron are strong permanent magnets .
- Praseodymium become the dyeing of glass and enamel used (for example, in green colored headlight glass in lighting technology).
- Improve links the UV - absorption and are used for eye protection glasses during welding used.
links
Oxides
- green praseodymium (III) oxide (Pr 2 O 3 )
- brown-black praseodymium (III, IV) oxide (Pr 6 O 11 )
- almost black praseodymium (IV) oxide (PrO 2 )
Halides
Several halides of all oxidation levels are known, for example praseodymium (III) fluoride (PrF 3 ), praseodymium (IV) fluoride (PrF 4 ), praseodymium (III) chloride (PrCl 3 ), praseodymium (III) bromide (PrBr 3 ), Praseodymium (III) iodide (PrI 3 ), praseodymium (II, III) iodide (Pr 2 I 5 ). The trivalent halides form different hydrates .
It also forms several fluorido complexes such as B. the K 2 [PrF 6 ] with tetravalent Pr.
Other connections
Binary connections are e.g. B. Praseodymium (III) sulfide (Pr 2 S 3 ), praseodymium nitride (PrN), praseodymium phosphide (PrP).
In addition, praseodymium is in various salts, such as the hygroscopic praseodymium (III) nitrate (Pr (NO 3 ) 3 x H 2 O), the nicely crystallizing praseodymium (III) sulfate (Pr 2 (SO 4 ) 3 8 H 2 O) and others.
The category: Praseodymium compounds provides an overview of praseodymium compounds .
Web links
- Entry on praseodymium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
- Metallic praseodymium
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (praseodymium) , unless otherwise stated .
- ^ IUPAC, Standard Atomic Weights Revised v2 ( Memento of March 3, 2016 in the Internet Archive ).
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ a b c d e Entry on praseodymium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e Entry on praseodymium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 1579.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337; doi: 10.1021 / je1011086 .
- ↑ a b Datasheet Praseodymium, powder from Sigma-Aldrich , accessed on April 26, 2017 ( PDF ).
- ↑ Carl Auer v. Welsbach: The decomposition of the Didym into its elements. In: Monthly magazine for chemistry . 6 (1), 1885, pp. 477-491; doi: 10.1007 / BF01554643 .