Praseodymium (III) nitrate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
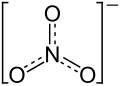
|
||||||||||
| General | ||||||||||
| Surname | Praseodymium (III) nitrate | |||||||||
| other names |
|
|||||||||
| Molecular formula |
|
|||||||||
| Brief description |
light green solid (hexahydrate) |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
≈56 ° C |
|||||||||
| solubility |
soluble in water, 80 g l −1 (22 ° C) (hexahydrate) |
|||||||||
| safety instructions | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Praseodymium (III) nitrate (Pr (NO 3 ) 3 ) is a salt of the rare earth metal praseodymium with nitric acid .
presentation
Crystals of the hexahydrate can be grown from solutions of Pr 6 O 11 in dilute nitric acid.
properties
Praseodymium (III) nitrate forms green crystals as hexahydrate . It is stable under normal conditions. At elevated temperatures it gradually loses its water of crystallization , above 253 ° C the anhydrous form is retained. From approx. 310 ° C, decomposition to praseodymium oxide nitrate (PrONO 3 ) and nitrogen oxides takes place.
Individual evidence
- ^ A b F. J. Rey, J. Martín-Gil, A. Gonzáles, FJ Martín-Gil: Kinetic analysis of thermal decomposition of praseodymium (III) nitrate hexahydrate . In: Journal of thermal analysis . tape 35 , no. 3 , May 1, 1989, ISSN 1572-8943 , pp. 805-813 , doi : 10.1007 / BF02057236 .
- ↑ Sigma-Aldrich: Safety data sheet . Retrieved April 8, 2020 .
- ↑ a b c J. Newton Friend: 346. The nitrates of neodymium and praseodymium, and their solubilities in water . In: Journal of the Chemical Society (Resumed) . 1935, ISSN 0368-1769 , p. 1430 , doi : 10.1039 / jr9350001430 .
- ↑ a b PubChem: Praseodymium nitrate. Retrieved April 8, 2020 .




