Einsteinium
| properties | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Einsteinium, Es, 99 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Actinoids | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | Ac , 7 , f | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7429-92-7 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 252 and | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius | 203 pm | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Rn ] 5 f 11 7 s 2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 6th.36758 (25) eV ≈ 614.38 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 12.2 (4) eV ≈ 1 180 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 22nd.7 (4) eV ≈ 2 190 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 38.8 (4) eV ≈ 3 740 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 54.1 (1.9) eV ≈ 5 220 kJ / mol | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Modifications | 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Crystal structure | cubic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | (calculated) 8.84 g / cm 3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 1133 K (860 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 1269 K (996 ° C) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +2, +3 , (+4) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | (Es 3+ + 3 e - → Es) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Hazard and safety information | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 Radioactive |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Einsteinium is an exclusively artificially produced chemical element with the element symbol Es and the atomic number 99. In the periodic table it is in the group of actinides ( 7th period , f-block ) and is one of the transuranic elements . Einsteinium is a radioactive metal, which can only be produced with great effort in just weighable quantities. It was discovered in 1952 after the test of the first American hydrogen bomb and named in honor of Albert Einstein , who, however, had nothing to do with the discovery or research of Einsteinium. It is produced in very small quantities in nuclear reactors . The metal and its compounds are obtained in small quantities primarily for study purposes.
history


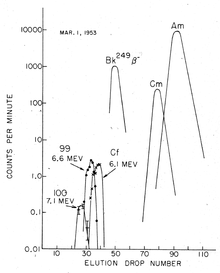
Einsteinium was found along with fermium after testing the first American hydrogen bomb, Ivy Mike , on November 1, 1952 on the Eniwetok Atoll . The first samples were obtained on filter papers that were carried when flying through the explosion cloud. Larger amounts were later isolated from corals. For reasons of military secrecy, the results were initially not published.
An initial investigation of the remains of the explosion had shown the formation of a new plutonium isotope 244 Pu, which could only have resulted from the uptake of six neutrons by a uranium- 238 core and two subsequent β-decays .
At the time, it was believed that the absorption of neutrons by a heavy nucleus was a rare occurrence. However, the identification of 244 Pu led to the conclusion that uranium nuclei can capture many neutrons, resulting in new elements.
The dissolved actinide ions were separated in the presence of a citric acid / ammonium citrate buffer in a weakly acidic medium ( pH ≈ 3.5) with ion exchangers at an elevated temperature. Element 99 (Einsteinium) was quickly detected; the isotope 253 Es, a high-energy α-emitter (6.6 MeV), was first found. It is created by capturing 15 neutrons from 238 U, followed by seven β decays.
This formation through continued neutron capture was possible because at the moment of detonation the neutron flux density was so high that most of the - radioactive - atomic nuclei that had formed in the meantime had not decayed by the next neutron capture. With a very high neutron flux, the mass number increases sharply without the atomic number changing. Only then do the resulting unstable nuclides decay over many β-decays to stable or unstable nuclides with a high atomic number:
In September 1953, there was no telling when the results of the teams in Berkeley , Argonne and Los Alamos would be published. It was decided to produce the new elements through bombardment experiments; At the same time, it was assured that these results would not fall under secrecy and could therefore be published. Einsteinium isotopes were produced shortly afterwards at the University of California Radiation Laboratory by bombarding uranium ( 238 U) with nitrogen ( 14 N). It was noted that there is research on this element that has so far been kept secret. Isotopes of the two newly discovered elements were generated by irradiating the plutonium isotope 239 Pu, and the results were published in five publications in quick succession. The final reactions from Californium are:
The Berkeley team was also concerned that another group of researchers might discover and publish the lighter isotopes of element 100 by ion bombardment before they could publish their classified research. Because at the end of 1953 and at the beginning of 1954 a working group from the Nobel Institute for Physics in Stockholm fired at uranium nuclei with oxygen nuclei; the isotope with the mass number 250 of the element 100 ( 250 μm) was formed.
The Berkeley team has already published some results on the chemical properties of both elements. Finally, the results of the thermonuclear explosion were released in 1955 and then published.
Ultimately, the Berkeley team's priority was universally recognized, as their five publications preceded the Swedish publication and they could rely on the previously secret results of the 1952 thermonuclear explosion. This was associated with the privilege of naming the new elements. They decided to name them after famous scientists who had already died. It was quickly agreed to give the names in honor of Albert Einstein and Enrico Fermi , both of whom had recently died: “We suggest for the name for the element with the atomic number 99, einsteinium (symbol E) after Albert Einstein and for the name for the element with atomic number 100, fermium (symbol Fm), after Enrico Fermi. "The announcement for the two newly discovered elements Einsteinium and Fermium was made by Albert Ghiorso at the 1st Geneva Atomic Conference , which took place from 8 to Took place on August 20, 1955. The element symbol for Einsteinium was later changed from E to Es .
Isotopes
All 17 nuclides and 3 nuclear isomers known to date are radioactive and unstable. The known mass numbers ranging from 241 to 258. The longest half-life has the isotope 252 may making it on earth no natural deposits give more with 471.7 days. 254 It has a half-life of 275.7 days, 255 Es of 39.8 days and 253 Es of 20.47 days. All other radioactive isotopes have half-lives below 40 hours, the majority of them are below 30 minutes. Of the 3 core isomers, 254 m Es is the most stable with t ½ = 39.3 hours.
→ List of Einsteinium isotopes
Extraction and presentation
Einsteinium is created by bombarding lighter actinides with neutrons in a nuclear reactor. The main source is the 85 MW high-flux isotope reactor at Oak Ridge National Laboratory in Tennessee, USA, which is set up for the production of transcurium elements (Z> 96).
In 1961 enough Einsteinium was synthesized to produce a weighable amount of the isotope 253 Es. This sample weighed about 0.01 mg and was used to make Mendelevium . Another Einsteinium was made at Oak Ridge National Laboratory by bombarding 239 Pu with neutrons . Approximately 3 milligrams were obtained in four years of continuous irradiation from one kilogram of plutonium and subsequent separation.
Extraction of Einsteinium isotopes
Small amounts of einsteinium and fermium were isolated and separated from plutonium , which was irradiated with neutrons. Four Einsteinium isotopes were found (with details of the half-lives measured at the time):
- 253 Es: α-emitters with t ½ = 20.03 days and with a spontaneous fission half-life of 7 × 10 5 years
- 254 m Es: β-emitter with t ½ = 38.5 hours
- 254 Es: α emitters with t ½ ≈ 320 days
- 255 Es: β-emitters with t ½ = 24 days
Two fermium isotopes were found:
- 254 Fm: α-emitters with t ½ = 3.24 hours and with a spontaneous fission half-life of 246 days
- 255 Fm: α-emitter with t ½ = 21.5 hours
Bombarding uranium with five-fold ionized nitrogen and six-fold ionized oxygen atoms also produced Einsteinium and Fermium isotopes.
The isotope 248 Es was identified when 249 Cf was bombarded with deuterium. It decays mainly through electron capture (ε) with a half-life of 25 ± 5 minutes but also through the emission of α-particles (6.87 ± 0.02 MeV). The ratio (ε / α) of ∼ 400 could be identified by the amount of 248 Cf generated by electron capture .
The isotopes 249 Es, 250 Es, 251 Es and 252 Es were generated by bombarding 249 Bk with α-particles. Thereby 4 to 1 neutrons can leave the nucleus, so that the formation of four different isotopes is possible.
Although the isotope 252 Es has the longest half-life, the isotope 253 Es is more easily accessible and is mainly used to determine the chemical properties. It was obtained by irradiating 100 to 200 μg 252 Cf with thermal neutrons (flux density: 2 to 5 × 10 14 neutrons × cm −2 s −1 , time period: 500 to 900 h). Ammonium α-hydroxyisobutyrate was used for the separation .
Depiction of elementary Einsteinium
Einsteinium is obtained by reducing Einsteinium (III) fluoride with lithium or Einsteinium (III) oxide with lanthanum .
properties
In the periodic table , the einsteinium with atomic number 99 is in the actinoid series, its predecessor is the californium, the following element is the fermium. Its analogue in the series of lanthanides is the holmium .
Physical Properties
Einsteinium is a radioactive metal with a melting point of 860 ° C, a boiling point of 996 ° C and a density of 8.84 g / cm 3 . It crystallizes in the cubic crystal system in the space group Fm 3 m (space group no. 225) with the lattice parameter a = 575 pm, which corresponds to a face-centered cubic lattice (fcc) or a cubic closest packing of spheres with the stacking sequence ABC. The radioactivity is so strong that it destroys the metal grid. The metal is divalent and has a noticeably high volatility.
Chemical properties
Like all actinides, Einsteinium is very reactive. The trivalent oxidation state is most stable in aqueous solution , but bivalent and tetravalent compounds are also known. Bivalent compounds could already be represented as solids; the tetravalent state could already be postulated during chemical transport in tracer quantities, but a final confirmation is still pending. Aqueous solutions with Es 3+ ions are pale pink in color.
safety instructions
Classifications according to the CLP regulation are not available because they only include chemical hazard and play a completely subordinate role compared to the hazards based on radioactivity . The latter also only applies if the amount of substance involved is relevant.
use
Einsteinium is mainly used in the production of higher transuranic elements and transactinides . Otherwise, the metal and its compounds are primarily obtained in small quantities for study purposes.
links
→ Category: Einsteinium connection
The study of einsteinium compounds is limited by several factors:
- The most readily available isotope 253 It is only available once or twice a year on a microgram scale.
- Long- range orders in solids are very quickly destroyed by the intense alpha radiation generated in around 20 days' half-life .
- The radioactive decay produces the isotopes 249 Bk and 249 Cf, which quickly contaminate the sample. The rate is around 3% per day.
Oxides
Einsteinium (III) oxide (Es 2 O 3 ) was obtained by calcining the corresponding nitrate in submicrogram quantities. The lattice parameter of the body-centered cubic crystal is 1076.6 (6) pm . A monoclinic and a hexagonal lanthanum (III) oxide structure are also known.
Oxyhalides
The oxyhalides Einsteinium (III) oxychloride (EsOCl), Einsteinium (III) oxibromide (EsOBr) and Einsteinium (III) oxyiodide (EsOI) are known. Einsteinium (III) oxychloride has a tetragonal structure of the PbFCl type.
Halides
Halides are known to have the +2 and +3 oxidation states. The most stable level +3 is known for all compounds from fluorine to iodine and is also stable in aqueous solution.
| Oxidation number | F. | Cl | Br | I. |
| +3 |
Einsteinium (III) fluoride EsF 3 |
Einsteinium (III) chloride EsCl 3 orange |
Einsteinium (III) bromide EsBr 3 white-yellow |
Einsteinium (III) iodide EsI 3 amber colored |
| +2 |
Einsteinium (II) chloride EsCl 2 |
Einsteinium (II) bromide EsBr 2 |
Einsteinium (II) iodide EsI 2 |
Einsteinium (III) fluoride (EsF 3 ) can be prepared by precipitation from Einsteinium (III) chloride solutions with fluoride, as well as from Einsteinium (III) oxide by reaction with ClF 3 or F 2 at 1-2 atmospheres pressure and 300-400 ° C. The crystal structure could not be determined, but it is assumed that it corresponds to the LaF 3 type , as with Berkelium (III) fluoride (BkF 3 ) and Californium (III) fluoride (CfF 3 ) .
Einsteinium (III) chloride (EsCl 3 ) is an orange-colored solid and forms a hexagonal structure of the UCl 3 type , with the Es atom being coordinated 9 times.
Einsteinium (III) bromide (EsBr 3 ) is a white-yellow solid and forms a monoclinic structure of the AlCl 3 type .
Einsteinium (III) iodide (EsI 3 ) is an amber-colored solid and forms a hexagonal structure of the BiI 3 type .
The divalent compounds of Einsteinium are produced by reducing the trivalent halides with hydrogen .
For einsteinium (II) chloride (EsCl 2 ), einsteinium (II) bromide (EsBr 2 ) and einsteinium (II) iodide (EsI 2 ), no detailed crystallographic data are known, but measured values of absorption bands are known.
literature
- Richard G. Haire: Einsteinium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1577-1620 ( doi: 10.1007 / 1-4020-3598-5_12 ).
- Glenn T. Seaborg (Ed.): Proceedings of the Symposium Commemorating the 25th Anniversary of Elements 99 and 100 , January 23, 1978; Report LBL-7701, April 1979.
- Gmelin's Handbook of Inorganic Chemistry , System No. 71, Transurane: Part A 1 II, pp. 20-21; Part A 2, pp. 46-47; Part B1, pp. 82-84.
Web links
- Entry to Einsteinium. In: Römpp Online . Georg Thieme Verlag, accessed on January 3, 2015.
- Albert Ghiorso: Einsteinium and Fermium , Chemical & Engineering News, 2003.
Individual evidence
- ↑ The values of the atomic and physical properties (Infobox) are, unless otherwise stated, taken from: Richard G. Haire: Einsteinium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (Ed.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1577-1620.
- ↑ a b c d e Entry on einsteinium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 13, 2020.
- ↑ a b c d e entry on einsteinium at WebElements, https://www.webelements.com , accessed on June 13, 2020.
- ↑ The hazards emanating from radioactivity do not belong to the properties to be classified according to the GHS labeling. With regard to other hazards, this element has either not yet been classified or a reliable and citable source has not yet been found.
- ↑ a b c d e f g h i Albert Ghiorso: Einsteinium and Fermium , Chemical & Engineering News, 2003.
- ↑ Albert Ghiorso , G. Bernard Rossi, Bernard G. Harvey, Stanley G. Thompson: Reactions of U 238 with Cyclotron-Produced Nitrogen Ions , in: Physical Review , 1954 , 93 (1), pp. 257-257 ( doi: 10.1103 / PhysRev.93.257 ).
- ↑ SG Thompson, A. Ghiorso, BG Harvey, GR Choppin: Transcurium Isotopes Produced in the Neutron Irradiation of Plutonium , in: Physical Review , 1954 , 93 (4), pp. 908-908 ( doi: 10.1103 / PhysRev.93.908 ) .
- ↑ BG Harvey, SG Thompson, A. Ghiorso, GR Choppin: Further Production of Transcurium Nuclides by Neutron Irradiation , in: Physical Review , 1954 , 93 (5), pp. 1129-1129 ( doi: 10.1103 / PhysRev.93.1129 ).
- ↑ MH Studier, PR Fields, H. Diamond, JF Mech, AM Friedman, PA Sellers, G. Pyle, CM Stevens, LB Magnusson, JR Huizenga: Elements 99 and 100 from Pile-Irradiated Plutonium , in: Physical Review , 1954 , 93 (6), pp. 1428-1428 ( doi: 10.1103 / PhysRev.93.1428 ).
- ↑ PR Fields, MH Studier, JF Mech, H. Diamond, AM Friedman, LB Magnusson, JR Huizenga: Additional Properties of Isotopes of Elements 99 and 100 , in: Physical Review , 1954 , 94 (1), pp. 209-210 ( doi: 10.1103 / PhysRev.94.209 ).
- ↑ GR Choppin, SG Thompson, A. Ghiorso, BG Harvey: Nuclear Properties of Some Isotopes of Californium, Elements 99 and 100 , in: Physical Review , 1954 , 94 (4), pp. 1080-1081 ( doi: 10.1103 / PhysRev .94.1080 ).
- ↑ Hugo Atterling, Wilhelm Forsling, Lennart W. Holm, Lars Melander, Björn Åström: Element 100 Produced by Means of Cyclotron-Accelerated Oxygen Ions , in: Physical Review , 1954 , 95 (2), pp. 585-586 ( doi: 10.1103 / PhysRev.95.585.2 ).
- ^ GT Seaborg , SG Thompson, BG Harvey, GR Choppin: Chemical Properties of Elements 99 and 100 ( abstract ; machine script (July 23, 1954), Radiation Laboratory, University of California, Berkeley, UCRL-2591 (Rev.) ).
- ^ SG Thompson, BG Harvey, GR Choppin, GT Seaborg: Chemical Properties of Elements 99 and 100 , in: J. Am. Chem. Soc. , 1954 , 76 (24), pp. 6229-6236 ( doi: 10.1021 / ja01653a004 ).
- ^ A b A. Ghiorso, SG Thompson, GH Higgins, GT Seaborg ( Radiation Laboratory and Department of Chemistry, University of California, Berkeley, California ), MH Studier, PR Fields, SM Fried, H. Diamond, JF Mech, GL Pyle , JR Huizenga, A. Hirsch, WM Manning ( Argonne National Laboratory, Lemont, Illinois ), CI Browne, HL Smith, RW Spence ( Los Alamos Scientific Laboratory, Los Alamos, New Mexico ): New Elements Einsteinium and Fermium, Atomic Numbers 99 and 100 , in: Physical Review , 1955 , 99 (3), pp. 1048-1049 ( doi: 10.1103 / PhysRev.99.1048 ; Maschinoscript (June 9, 1955), Lawrence Berkeley National Laboratory. Paper UCRL-3036 ).
- ^ PR Fields, MH Studier, H. Diamond, JF Mech, MG Inghram, GL Pyle, CM Stevens, S. Fried, WM Manning ( Argonne National Laboratory, Lemont, Illinois ); A. Ghiorso, SG Thompson, GH Higgins, GT Seaborg ( University of California, Berkeley, California ): Transplutonium Elements in Thermonuclear Test Debris , in: Physical Review , 1956 , 102 (1), pp. 180-182 ( doi: 10.1103 /PhysRev.102.180 ).
- ↑ a b Richard G. Haire: Einsteinium , in: Lester R. Morss, Norman M. Edelstein, Jean Fuger (eds.): The Chemistry of the Actinide and Transactinide Elements , Springer, Dordrecht 2006; ISBN 1-4020-3555-1 , pp. 1577-1620.
- ↑ G. Pfennig, H. Klewe-Nebenius, W. Seelmann-Eggebert (eds.): Karlsruher Nuklidkarte , 7th edition, 2006.
- ↑ Irshad Ahmad, Frank Wagner, Jr .: Half-life of the longest-lived Einsteinium Isotope- 252 Es , in: J. Inorg. Nucl. Chem. , 1977 , 39 (9), pp. 1509-1511 ( doi: 10.1016 / 0022-1902 (77) 80089-4 ).
- ↑ William McHarris, FS Stephens, F. Asaro, I. Perlman: Decay scheme of einsteinium-254 , in: Physical Review , 1966 , 144 (3), pp 1031-1045 ( doi: 10.1103 / PhysRev.144.1031 ).
- ↑ G. Audi, O. Bersillon, J. Blachot, AH Wapstra: The NUBASE evaluation of nuclear and decay properties , in: Nuclear Physics A , 729, 2003, pp. 3–128. doi : 10.1016 / j.nuclphysa.2003.11.001 . ( PDF ; 1.0 MB).
- ^ High Flux Isotope Reactor , Oak Ridge National Laboratory; Retrieved September 23, 2010.
- ↑ Darleane C. Hoffman, Albert Ghiorso, Glenn Theodore Seaborg: The Transuranium People: The Inside Story , Imperial College Press, 2000, ISBN 978-1-86094-087-3 , pp. 190–191 ( limited preview in Google Book search).
- ↑ M. Jones, RP Schuman, JP Butler, G. Cowper, TA Eastwood, HG Jackson: Isotopes of Einsteinium and Fermium Produced by Neutron Irradiation of Plutonium , in: Physical Review , 1956 , 102 (1), pp. 203-207 ( doi: 10.1103 / PhysRev.102.203 ).
- ↑ LI Guseva, KV Filippova, Yu. B. Gerlit, VA Druin, BF Myasoedov, NI Tarantin: Experiments on the Production of Einsteinium and Fermium with a Cyclotron , in: Journal of Nuclear Energy , 1954 , 3 (4), pp. 341–346 (translated in November 1956) ( doi: 10.1016 / 0891-3919 (56) 90064-X ).
- ↑ A. Chetham-Strode, LW Holm: New Isotope Einsteinium-248 , in: Physical Review , 1956 , 104 (5), pp. 1314-1314 ( doi: 10.1103 / PhysRev.104.1314 ).
- ^ Bernard G. Harvey, Alfred Chetham-Strode, Albert Ghiorso, Gregory R. Choppin, Stanley G. Thompson: New Isotopes of Einsteinium , in: Physical Review , 1956 , 104 (5), pp. 1315-1319 ( doi: 10.1103 /PhysRev.104.1315 ).
- ↑ SA Kulyukhin, LN Auerman, VL Novichenko, NB Mikheev, IA Rumer, AN Kamenskaya, LA Goncharov, AI Smirnov: Production of Microgram Quantities of Einsteinium-253 by the Reactor Irradiation of Californium , in: Inorganica Chimica Acta , 1985 , 110 ( 1), pp. 25-26 ( doi: 10.1016 / S0020-1693 (00) 81347-X ).
- ↑ BB Cunningham, TC Parsons, USAEC Doc. UCRL-20426 (1971), p. 239.
- ^ A b R. G. Haire, RD Baybarz: Studies of einsteinium metal , in: Journal de Physique Colloques , 1979 , 40 (C4), pp. 101-102 ( doi: 10.1051 / jphyscol: 1979431 ; PDF ; PDF ( machine script ) ).
- ^ RG Haire: Properties of the Transplutonium Metals (Am-Fm) , in: Metals Handbook, Vol. 2, 10th Edition, (ASM International, Materials Park, Ohio, 1990), pp. 1198-1201.
- ↑ Phillip D. Kleinschmidt, John W. Ward, George M. Matlack, Richard G. Haire: Henry's Law vaporization studies and thermodynamics of einsteinium ‐ 253 metal dissolved in ytterbium , in: J. Chem. Phys. , 1984 , 81 , pp. 473-477 ( doi: 10.1063 / 1.447328 ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1956.
- ^ A b D. D. Ensor, JR Peterson, RG Haire, JP Young: Absorption spectrophotometric study of 253 EsF 3 and its decay products in the bulk-phase solid state , in: J. Inorg. Nucl. Chem. , 1981 , 43 (10), pp. 2425-2427 ( doi: 10.1016 / 0022-1902 (81) 80274-6 ).
- ↑ a b R. G. Haire, RD Baybarz: Identification and Analysis of Einsteinium Sesquioxide by Electron Diffraction , in: J. Inorg. Nucl. Chem. , 1973 , 35 (2), pp. 489-496 ( doi: 10.1016 / 0022-1902 (73) 80561-5 ).
- ↑ RG Haire, L. Eyring, in: Handbook on the Physics and Chemistry of Rare Earths , vol. 18 Lanthanoids and Actinides Chemistry (ed. By KA Gscheidner, Jr., L. Eyring, GR Choppin, GH Lander), North-Holland, New York 1994, pp. 414-505.
- Jump up ↑ a b c d e J. P. Young, RG Haire, JR Peterson, DD Ensor, RL Fellow: Chemical Consequences of Radioactive Decay. 2. Spectrophotometric Study of the Ingrowth of Berkelium-249 and Californium-249 into Halides of Einsteinium-253 , in: Inorg. Chem. , 1981 , 20 (11), pp. 3979-3983 ( doi: 10.1021 / ic50225a076 ).
- ^ A b J. R. Peterson, DD Ensor, RL Fellows, RG Haire, JP Young: Preparation, characterization, and decay of einsteinium (II) in the solid state , in: Journal de Physique Colloques , 1979 , 40 (C4), p. 111-113 ( doi: 10.1051 / jphyscol: 1979435 ; PDF ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1969.
- ↑ JP Young, RG Haire, RL Fellows, JR Peterson: Spectrophotometric studies of transcurium element halides and oxyhalides in the solid state , in: Journal of Radioanalytical Chemistry , 1978 , 43 (2), pp. 479-488 ( doi: 10.1007 / BF02519508 ).
- ↑ DK Fujita, BB Cunningham, TC Parsons, JR Peterson: The solution absorption spectrum of Es 3+ , in: Inorg. Nucl. Chem. Lett. , 1969 , 5 (4), pp. 245-250 ( doi: 10.1016 / 0020-1650 (69) 80192-3 ).
- ↑ DK Fujita, BB Cunningham, TC Parsons: Crystal structures and lattice parameters of einsteinium trichloride and einsteinium oxychloride , in: Inorg. Nucl. Chem. Lett. , 1969 , 5 (4), pp. 307-313 ( doi: 10.1016 / 0020-1650 (69) 80203-5 ).
- ↑ RL Fellows, JR Peterson, M. Noé, JP Young, RG Haire: X-ray diffraction and spectroscopic studies of crystalline einsteinium (III) bromide, 253 EsBr 3 , in: Inorg. Nucl. Chem. Lett. , 1975 , 11 (11), pp. 737-742 ( doi: 10.1016 / 0020-1650 (75) 80090-0 ).
- ^ RG Haire, ORNL Report 5485, 1978 .
- ^ JR Peterson: Chemical Properties of Einsteinium: Part II , in: GT Seaborg (Ed.): Proceedings of the 'Symposium Commemorating the 25th Anniversary of Elements 99 and 100' , January 23, 1978; Report LBL-7701, April 1979, pp. 55-64.
- ↑ RL Fellows, JP Young, RG Haire, JR Peterson, in: The Rare Earths in Modern Science and Technology (edited by GJ McCarthy and JJ Rhyne), Plenum Press, New York 1977, pp. 493-499.
- ↑ JP Young, RG Haire, RL Fellows, M. Noé, JR Peterson: Spectroscopic and X-ray Diffraction Studies of the Bromides of Cf-249 and Es-253 , in: Transplutonium 1975 (edited by W. Müller and R. Lindner), North Holland, Amsterdam 1976, pp. 227-234.
![\ mathrm {^ {238} _ {\ 92} U \ {\ xrightarrow [{- 2 \ \ beta ^ {-}}] {+ \ 6 \ (n, \ gamma)}} \ _ {\ 94} ^ {244} Pooh}](https://wikimedia.org/api/rest_v1/media/math/render/svg/c4b60ac4bf0d4ebf96b6a71fefa86f13dd5784e0)
![\ mathrm {^ {238} _ {\ 92} U \ {\ xrightarrow [{- 7 \ \ beta ^ {-}}] {+ \ 15, \ 16, \ 17 \ (n, \ gamma)}} \ _ {\ \ \ \ \ \ \ \ \ \ \ \ 99} ^ {253, \ 254, \ 255} Es}](https://wikimedia.org/api/rest_v1/media/math/render/svg/40a621970bb60c515339d427edbe5018ca880d2c)
![\ mathrm {^ {252} _ {\ 98} Cf \ {\ xrightarrow {(n, \ gamma)}} \ _ {\ 98} ^ {253} Cf \ {\ xrightarrow [{17.81 \ d}] {\ beta ^ {-}}} \ _ {\ 99} ^ {253} It \ {\ xrightarrow {(n, \ gamma)}} \ _ {\ 99} ^ {254} It \ {\ xrightarrow [{ }] {\ beta ^ {-}}} \ _ {100} ^ {254} Fm}](https://wikimedia.org/api/rest_v1/media/math/render/svg/2dcf5875c42aad904ed8c8a397de8fc552cf5f71)
![\ mathrm {^ {249} _ {\ 98} Cf \ + \ _ {1} ^ {2} D \ \ longrightarrow \ _ {\ 99} ^ {248} Es \ + \ 3 \ _ {0} ^ { 1} n \ quad \ left (^ {248} _ {\ 99} Es \ {\ xrightarrow [{27 \ min}] {\ epsilon}} \ _ {\ 98} ^ {248} Cf \ right)}](https://wikimedia.org/api/rest_v1/media/math/render/svg/85115ad812c3c66ccfd6c7741439fce1fc1be1c2)
![\ mathrm {^ {249} _ {\ 97} Bk \ {\ xrightarrow [{- \ 4 \ to \ 1 \ n}] {+ \ alpha}} \ _ {\ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ \ 99} ^ {249, \ 250, \ 251, \ 252} Es}](https://wikimedia.org/api/rest_v1/media/math/render/svg/16e216c81a272463e710f8aa9d0f10f819a0589c)
![\ mathrm {^ {252} _ {\ 98} Cf \ {\ xrightarrow {(n, \ gamma)}} \ _ {\ 98} ^ {253} Cf \ {\ xrightarrow [{17.81 \ d}] {\ beta ^ {-}}} \ _ {\ 99} ^ {253} Es}](https://wikimedia.org/api/rest_v1/media/math/render/svg/5f409bd2bd31e6e316748643fc25eb44d318bef5)



![\ mathrm {^ {253} _ {\ 99} Es \ {\ xrightarrow [{20 \ d}] {\ alpha}} \ _ {\ 97} ^ {249} Bk \ {\ xrightarrow [{314 \ d} ] {\ beta ^ {-}}} \ _ {\ 98} ^ {249} Cf}](https://wikimedia.org/api/rest_v1/media/math/render/svg/a2e6d53c027c0247a7ddbc0c45718d09789f2da2)

