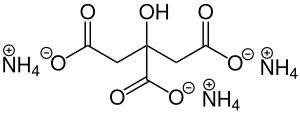
Ammonium citrate
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Ammonium citrate | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 17 N 3 O 7 | ||||||||||||||||||
| Brief description |
colorless, crystalline substance |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 243.22 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
1.48 g cm −3 (20 ° C) |
||||||||||||||||||
| Melting point |
185 ° C |
||||||||||||||||||
| solubility |
easy in water (1000 g l −1 at 20 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Ammonium citrate (NH 4 ) 3 (C 6 H 5 O 7 ) is the ammonium salt of citric acid . It consists of three ammonium ions (NH 4 ) + and the citrate ion (C 6 H 5 O 7 ) 3− and forms colorless crystals that dissolve well in water.
In addition to triammonium citrate, there is also monoammonium citrate NH 4 (C 6 H 7 O 7 ) and diammonium citrate (NH 4 ) 2 (C 6 H 6 O 7 ).
synthesis
Ammonium citrate can be obtained by neutralizing ammonia with citric acid:
properties
Triammonium citrate crystallizes in the orthorhombic crystal system with a = 6.223 Å, b = 15.048 Å and c = 11.056 Å.
use
Ammonium citrate is approved as a food additive E 380 in the EU . It is used as an acidity regulator and color stabilizer in lemonades, jams, canned fruit and vegetables, baked goods, ready-made soups and frozen fish.
Individual evidence
- ↑ Entry on E 380: Triammonium citrate in the European database for food additives, accessed on June 27, 2020.
- ↑ Data sheet ammonium citrate from AlfaAesar, accessed on March 26, 2010 ( PDF )(JavaScript required) .
- ↑ Entry on ammonium citrate, dibasic. In: Römpp Online . Georg Thieme Verlag, accessed on September 30, 2014.
- ↑ Ammonium citrate data sheet from Sigma-Aldrich , accessed on March 9, 2011 ( PDF ).
- ↑ a b c Entry on triammonium citrate in the GESTIS substance database of the IFA , accessed on February 10, 2017(JavaScript required) .
- ↑ External identifiers or database links for diammonium citrate: CAS number: 3012-65-5, EC number: 221-146-3, ECHA InfoCard: 100.019.225 , PubChem : 18171 , ChemSpider : 17164 , Wikidata : Q3678337 .
- ↑ M. Venkateshwarlu, T. Bhaskar Rao, K. Kishan Rao: Growth and characterization of triammonium citrate . In: Bulletin of Materials Science . tape 12 , no. 2 , 1989, pp. 143-146 , doi : 10.1007 / BF02744513 .


