Praseodymium (III) sulfate
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
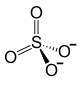
|
||||||||||
| General | ||||||||||
| Surname | Praseodymium (III) sulfate | |||||||||
| other names |
Praseodymium sulfate |
|||||||||
| Molecular formula |
|
|||||||||
| Brief description |
green monoclinic crystals |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | ||||||||||
| Physical state |
firmly |
|||||||||
| density |
2.83 g cm −3 (octahydrate) |
|||||||||
| solubility |
soluble in water, 170 g l −1 (20 ° C) (octahydrate) |
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
Praseodymium (III) sulfate (Pr 2 (SO 4 ) 3 ) is a salt of the rare earth metal praseodymium with sulfuric acid . As an octahydrate, it forms green monoclinic crystals.
presentation
Crystals of the octahydrate can be grown from solutions obtained by dissolving moistened Pr 2 O 3 powder with sulfuric acid. This process can be optimized by evaporation or dissolution steps with the participation of methanol and small amounts of 2,2'-bipyridine .
properties
Praseodymium (III) sulfate is stable under normal conditions. At elevated temperatures it gradually loses its water of crystallization , becoming pale and finally colorless as anhydrous Pr 2 (SO 4 ) 3 .
The octahydrate crystallizes monoclinically in the space group C 2 / c (space group no. 15) with the lattice parameters a = 1370 pm , b = 686 pm, c = 1845 pm and β = 102.8 ° as well as four formula units per unit cell . Like all rare earth metal sulfates, praseodymium sulfate is less soluble in hot water than in cold water. When a saturated solution is heated, a part crystallizes out.
A pentahydrate is also known.
Individual evidence
- ↑ a b c d David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Properties of the Elements and Inorganic Compounds, pp. 4-84.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b Y.-Q. Zheng, Y.-J. Zhu and J.-L. Lin: Redeterminaton of the crystal structure of praseodymium sulfate octahydrate, Pr 2 (SO 4 ) 3 · 8H 2 O . In: Journal of Crystallography - New Crystal Structures . 217, 2002, pp. 299-300. pdf doi : 10.1524 / ncrs.2002.217.jg.299
- ^ National Research Council (US): Bulletin of the National Research Council , 1919, National Academies, p. 97 ( limited preview in Google Book Search).

![{\ mathrm {\ \! \ {\ Biggr]} _ {2}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/574adab1409cb81da6c38bb738ad111e61bbb2d9)
![{\ mathrm {\ \! \ {\ Biggr]} _ {3}}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/cd32744e9f4314d390f5ee107a8bd812d0cc2da0)
