Sodium selenate
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
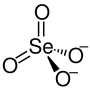
|
||||||||||||||||
| General | ||||||||||||||||
| Surname | Sodium selenate | |||||||||||||||
| other names |
Disodium tetraoxoselenate (IV) |
|||||||||||||||
| Molecular formula | Na 2 SeO 4 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 188.94 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
3.213 g cm −3 (17 ° C) |
|||||||||||||||
| solubility |
585 g l −1 at 25 ° C |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| Toxicological data | ||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Sodium selenate is an inorganic chemical compound of sodium from the selenate group .
Extraction and presentation
Sodium selenate can be obtained by reacting a selenic acid solution with sodium carbonate .
properties
Sodium selenate is a non-flammable, hygroscopic, white, odorless solid that is very easily soluble in water. It crystallizes orthorhombically , space group Fddd (space group no. 70) , with the lattice parameters a = 6.099 Å , b = 12.59 Å, c = 10.16 Å.
use
Sodium selenate is used as an insecticide and as an additive to fertilizers. It is also used in low doses as a dietary supplement and in medicine.
Individual evidence
- ↑ a b c d e f g Entry on sodium selenate in the GESTIS substance database of the IFA , accessed on January 8, 2018(JavaScript required) .
- ↑ David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Physical Constants of Inorganic Compounds, pp. 4-91.
- ↑ Georg Brauer (Ed.), With the collaboration of Marianne Baudler a . a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 425.
- ^ A. Kalman, DWJ Cruickshank: A note on the structure of Na 2 SeO 4 . In: Acta Crystallographica , B26, 1970, p. 436, doi: 10.1107 / S0567740870002534 .
- ↑ Ralf Steudel : Chemistry of Non-Metals: From Structure and Bond to Application . Walter de Gruyter, 2008, ISBN 3-11-021128-9 , p. 468 ( limited preview in Google Book search).
- ↑ Klaus Karl Degitz, Hanns-Juergen Krauss: pathogenesis, clinical and pharmacotherapy of acne . Govi-Verlag Eschborn, 2004, ISBN 3-7741-1021-2 ( limited preview in the Google book search).
- ↑ Frank Wappler, Gerald Spilker: Burn medicine: From the scene of an accident to rehabilitation . Georg Thieme Verlag, 2008, ISBN 3-13-159531-0 , p. 78 ( limited preview in Google Book search).
- ↑ Irmgard Niestroj: Practice of orthomolecular medicine: Physiological foundations. Therapy with ... Georg Thieme Verlag, 2000, ISBN 3-7773-1470-6 , p. 480 ( limited preview in Google Book Search).



