Diamagnetism
Diamagnetism is one of the manifestations of magnetism in matter . Diamagnetic materials develop an induced magnetic field in an external magnetic field in a direction opposite to the external magnetic field. Diamagnetic materials have a tendency to migrate out of an inhomogeneous magnetic field. Without an external magnetic field, diamagnetic materials do not have their own magnetic field; they are non-magnetic.
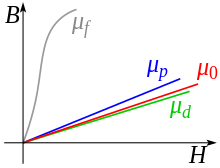
The proportionality factor of the field weakening is determined by the relative permeability (or the magnetic susceptibility ) and is less than 1 for diamagnets (see paramagnetism ).
In physics, all materials with negative magnetic susceptibility and without magnetic order are classified as diamagnetic. The most diamagnetic elements under normal conditions are bismuth and carbon .
history
In 1778, Sebald Justinus Brugmans observed that certain materials were repelled by magnetic fields. In 1845 Michael Faraday realized that all materials in nature react to external magnetic fields. He introduced the term "diamagnetism" into physics at the suggestion of the philosopher William Whewell .
model
When an external magnetic field H acts on diamagnetic materials, it changes the magnetic alignment of the constituent parts of the atoms in such a way that a magnetic moment arises which is opposite to the external magnetic field. The induced field B as the sum of the magnetic moments of the atoms of the material weakens this external field.
In the case of an inhomogeneous field, work has to be done to move a diamagnet into areas of higher field strength, since the compensating effects have to be intensified. A diamagnetic material naturally tends towards a lower field strength. The actual processes can only be explained quantum mechanically : the spin of each electron has a magnetic moment and thus generates a field which, however, due to the Pauli principle and the thermal movements, does not appear macroscopically. Only the external field induces rectified magnetic dipoles .
Based on these considerations, it becomes clear that any material is diamagnetic. However, because the diamagnetic effects are weaker than paramagnetism and orders of magnitude weaker than ferromagnetism , they only appear with materials that are neither para- nor ferromagnetic. Such substances are then called diamagnetic.
Diamagnetic materials have a magnetic susceptibility χ less than 0 or, accordingly, a relative permeability less than 1.
| material |
χ V (SI) also χ m or χ |
χ V (cgs) also χ m or χ |
χ mol (SI) m 3 · mol -1 |
χ mol (cgs) cm 3 mol −1 |
χ mass (SI) m³ · kg −1 |
χ mass (cgs) cm³ · g −1 |
|---|---|---|---|---|---|---|
| Aluminum (paramagnetic) | 2.1e-5 | 1.7the-6th | 2.1e-10 | 1.7the-5 | 7th.7the-9 | 6th.1e-7th |
| Aluminum sulfate anhydrous | -9.3e-6th | -7th.4the-7th | -1.2e-9 | -9.3e-5 | -3.4the-9 | -2.7the-7th |
| Aluminum sulfate · 18H 2 O | -1.6the-5 | -1.3e-6th | -4th.1e-9 | -3.2e-4th | -6th.1e-9 | -4th.8the-7th |
| beryllium | -2.3e-5 | -1.8the-6th | -1.1e-10 | -9.0e-6th | -1.3e-8th | -1.0e-6th |
| Bismuth | -1.7the-4th | -1.3e-5 | -3.5e-9 | -2.8the-4th | -1.7the-8th | -1.3e-6th |
| lead | -1.6the-5 | -1.3e-6th | -2.9e-10 | -2.3e-5 | -1.4the-9 | -1.1e-7th |
| boron | -1.9e-5 | -1.5e-6th | -8th.4the-11 | -6th.7the-6th | -7th.8the-9 | -6th.2e-7th |
| cadmium | -1.9e-5 | -1.5e-6th | -2.5e-10 | -2.0e-5 | -2.2e-9 | -1.8the-7th |
| Germanium | -7th.1e-5 | -5.6the-6th | -9.7the-10 | -7th.7the-5 | -1.3e-8th | -1.1e-6th |
| gold | -3.4the-5 | -2.7the-6th | -3.5e-10 | -2.8the-5 | -1.8the-9 | -1.4the-7th |
| Carbon (diamond) | -2.2e-5 | -1.7the-6th | -7th.4the-11 | -5.9e-6th | -6th.2e-9 | -4th.9e-7th |
| Carbon (pyrolytic graphite, vertical) | -4th.5e-4th | -3.6the-5 | -2.4the-9 | -1.9e-4th | -2.0e-7th | -1.6the-5 |
| Carbon (pyrolytic graphite, parallel) | -8th.5e-5 | -6th.8the-6th | -4th.5e-10 | -3.6the-5 | -3.8the-8th | -3.0e-6th |
| copper | -9.6the-6th | -7th.7the-7th | -6th.9e-11 | -5.5e-6th | -1.1e-9 | -8th.6the-8th |
| silver | -2.4the-5 | -1.9e-6th | -2.5e-10 | -2.0e-5 | -2.3e-9 | -1.8the-7th |
| water | -9.1e-6th | -7th.2e-7th | -1.6the-10 | -1.3e-5 | -9.1e-9 | -7th.2e-7th |
| zinc | -1.6the-5 | -1.2e-6th | -1.4the-10 | -1.1e-5 | -2.2e-9 | -1.7the-7th |
The susceptibility is only slightly dependent on the temperature, but often strongly on the physical state, the crystal system and the direction of the crystal lattice. A large anisotropy can be observed, for example, with pyrolytically deposited graphite (see sortable table). Compounds of paramagnetic elements, such as the aluminum listed here, can be diamagnetic.
Effects
Superconductor
Superconductors are perfect diamagnets with a susceptibility of −1: they displace the magnetic field lines from their interior ( Meißner-Ochsenfeld effect ).
Hover
The effect of wandering out of a magnetic field makes it possible, with a sufficiently strong magnetic field (around 15 Tesla in the laboratory), to let water and even living beings float. This effect is also called diamagnetic levitation ; Experiments with a hovering frog, a spider or a block of wood became known.
Pyrolytic graphite is strongly diamagnetic orthogonal to the crystal plane. With a strong neodymium magnet , graphite can be kept in suspension.
See also
- Paramagnetism
- Ferromagnetism
- Antiferromagnetism
- Ferrimagnetism
- Pyromagnetism
- Levitation (technique)
- Larmor diamagnetism
literature
- Hans Fischer: Materials in electrical engineering. 2nd Edition. Carl Hanser Verlag, Munich / Vienna 1982, ISBN 3-446-13553-7 .
- Horst Stöcker: Pocket book of physics. 4th edition. Verlag Harry Deutsch, Frankfurt am Main 2000, ISBN 3-8171-1628-4 .
- Günter Springer: Expertise in electrical engineering. 18th edition. Verlag Europa-Lehrmittel, Wuppertal 1989, ISBN 3-8085-3018-9 .
- LN Mulay, EA Boudreaux: Theory and applications of molecular diamagnetism. Wiley, New York 1976, ISBN 0-471-62358-X .
- Jakov Grigoŕevič Dorfman: Diamagnetism and Chemical Bonding. Teubner, Leipzig 1964, DNB 57290178X .
Web links
Individual evidence
- ↑ a b c d e f g h i j k l m n o p CRC Handbook of Chemistry and Physics. Chemical Rubber Publishing Company, Boca Raton 1990, ISBN 0-8493-0471-7 , pp. E-129 to E-145.
- ↑ a b c M. D. Simon, AK Geim: Diamagnetic levitation: Flying frogs and floating magnets. In: Journal of Applied Physics , 87, 2000, pp. 6200-6204, doi: 10.1063 / 1.372654 .