Fission product
The substances created by nuclear fission are called fission products . They arise in large quantities in nuclear reactors . Some of the radionuclides contained in fission products have useful applications, but safe disposal is important for the majority.
Fission products are not to be confused with neutron capture products such as plutonium , which are also produced from nuclear fuel in reactors.
Physical basics

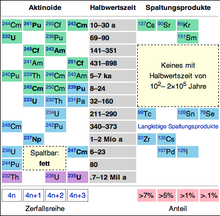
The immediately resulting (so-called primary ) fission product atomic nuclei or fission fragments have a large excess of neutrons ; they are therefore unstable ( radioactive ) and are transformed into other atomic nuclei through successive beta-minus decays . Since the mass number of the atomic nucleus remains the same during beta decay, the nuclides that arise one after the other form so-called isobaric chains . Each of these chains of decay continues up to a stable nuclide. These end products of nuclear fission are essentially metals with mass numbers around 90 (in the periodic table from zirconium to palladium) and some elements with mass numbers around 140 (tellurium to samarium; with the exception of tellurium, iodine and xenon also metals).
Most of these radioactive decays take place shortly (fractions of a second to hours) after the nuclear fission. The energy released in the process contributes several percent to the performance of a nuclear reactor ( decay heat ). Particularly important from a health point of view are those fission products in which the last (sometimes the penultimate) of these decays has a half-life of days to years, especially if, due to their chemical properties, they are easily transported and can get into the human body.
Some fission products have a significant influence on the functioning of a nuclear reactor. In particular, the cleavage products that release neutrons only delayed and only possible at all, to a reactor control , and nuclides that neutron strongly absorb , especially 135 Xe .
Exact numerical values for the yield of the various isobaric chains in the cleavage are given in a data collection of the International Atomic Energy Agency.
The initial composition of the resulting mixture of fission products depends on the type of reactor (fission material, neutron energy spectrum) and the length of time the fission products remain in the reactor (duration of further neutron irradiation). After removal from the reactor, the half-life and decomposition products of the individual fission products determine the change in composition over time.
Fission products can be gaseous (e.g. 133 Xe , 85 Kr ), volatile (e.g. 131 I ) or solid (e.g. 137 Cs , 90 Sr ).
Nuclear fission
Example of a neutron-induced nuclear fission of uranium-235:
Decay series of the primary fission products:
The stable end products Ru-101 and Cs-133 are formed by multiple beta decays.
The neutrons released during the fission - two in the example - are slowed down by collisions with the atomic nuclei of the surrounding matter and finally absorbed, ie "consumed" in a nuclear reaction (usually a neutron capture ).
Properties of selected fission products
The most common fission products from light water reactors are isotopes of iodine, cesium, strontium, xenon and barium . Many fission products break down quickly into stable nuclides, but a significant remainder have half-lives of more than a day, up to millions of years. Some nuclides with short to medium half-lives are used in medicine or industry. It is remarkable that not a single one of the nuclides produced during nuclear fission has a half-life of between 100 and 200,000 years. This gap leads to the fact that the total activity of the mixture of fission products decreases significantly in the first centuries, essentially until the isotope 137 Cs, which dominates during this period, has decayed. Thereafter, the residual activity (which is of course lower by orders of magnitude than the activity of the shorter-lived nuclides) remains practically unchanged over historical periods (many millennia). However, in addition to the fission products, there are also transuranic elements in spent fuel rods , which close this “gap” in the half-lives.
Cesium
Three cesium isotopes are found among the fission products . 134 Cs (half-life approx. 2 years) does not arise directly because the decay series of nuclei with mass number 134 ends with the stable nuclide 134 Xe, but indirectly through neutron capture from the stable fission product 133 Cs. 135 Cs has a very long half-life (2.3 million years) and is therefore only moderately radioactive. The most important cesium isotope is 137 Cs with a half-life of approx. 30 years. After the decay of the short-lived isotopes, it is the most strongly radiating nuclide in the mixture of fission products for many centuries.
Technetium
After the decay of 137 Cs, 99 Tc (half-life 211,100 years) is the most strongly radiating isotope of the remaining fission products (there are a total of seven fission products with very long half-lives). Technetium has no stable isotope and therefore hardly occurs in nature.
Along with 129 I, 99 Tc is a main candidate for transmutation ; its elimination would reduce radiation by approx. 90% in the distant future (after the decay of the shorter-lived isotopes).
krypton
Radioactive krypton is produced in small quantities in nuclear reactors. During the reprocessing of spent fissile material, for example to extract plutonium and remaining uranium, the relatively long-lived krypton isotope 85 Kr (half-life 10.756 years) is released and escapes into the atmosphere. The amount of radioactive krypton in the earth's atmosphere therefore gives an indication of the amount of processed fissile material. The difference between the krypton resulting from known processing and the measured krypton makes it possible to estimate the amount of processing that has been kept secret (usually for the manufacture of nuclear weapons ).
strontium
Since strontium has a biologically similar effect to calcium , the radioactive strontium isotope 90 Sr is one of the most potentially harmful fission products, because after being absorbed into the organism it is deposited in the bones and remains in the body until it decays. Since the half-life is about 30 years, you carry the strontium for the rest of your life. The really dangerous thing about 90 Sr is the daughter nuclide 90 Y, which also decays, whereby its beta radiation has four times the energy of that of 90 Sr.
The preferred absorption of strontium in the bones is used therapeutically or palliatively in bone cancer : Due to the preferred storage in the bone, strontium 89 Sr (half-life 50.5 days) can be used to combat daughter tumors .
Iodine
During nuclear fission, the iodine isotopes 127 I (0.12% of all fission when 235 U is fissioned in a thermal reactor), 129 I (0.7%) and 131 I (2.9%) are produced in varying amounts . 127 Iodine is not radioactive (natural iodine consists exclusively of 127 I); 129 Iodine is only weakly radioactive because of its long half-life (15.7 million years). 131 I , on the other hand, poses a particular risk , since iodine is very mobile in the environment due to its physico-chemical properties - it is particularly easily released in the event of an accident - and is also actively absorbed by the human organism as an essential trace element. The thyroid gland in particular contains high levels of iodine.
131 I has a half-life of 8 days. After 8 days, the radiation has decreased to half, after 27 days to a tenth and after 80 days to a thousandth. After the Chernobyl disaster , 131 I represented the dominant radioisotope in the first few days. With timely advance warning, a certain protection can be built up against a feared exposure by taking stable iodine in the form of potassium iodide tablets. This saturates the organism with iodine and then absorbs the radioactive iodine in significantly smaller quantities ( iodine blockade ).
Ruthenium, rhodium and palladium
Ruthenium and rhodium are among the most common elements in the mixture of fission products (11% and 3% in the case of the fission of 235 U in a thermal reactor). Separation and recycling of these valuable precious metals in the context of reprocessing is conceivable, but has not yet been practiced. Ruthenium could only be used after a waiting period of several years, since the isotope mixture produced in the reactor initially also contains the radioactive 106 Ru (half-life 374 days).
Palladium is formed in somewhat smaller quantities (1.6% when 235 U is cleaved). In addition to the stable isotopes 105 Pd, 106 Pd, 108 Pd and 110 Pd, it also contains the slightly radioactive 107 Pd (half-life 6.5 million years), which severely limits its usability.
Individual evidence
literature
- K. Kugeler, P.-W. Phlippen: energy technology. Technical, economic and ecological basics. Springer-Verlag 1990. ISBN 978-3-540-52865-4
- J. Hala, JD Navratil: Radioactivity, Ionizing Radiation and Nuclear Energy. Konvoj, Brno 2003. ISBN 80-7302-053-X .


![{\ mathrm {^ {{133}} _ {{\ 52}} Te \ {\ xrightarrow [{{12.5} \ {\ min}}] {\ beta ^ {{-}}}} \ _ { {\ 53}} ^ {{133}} I \ {\ xrightarrow [{{20.8} \ {\ h}}] {\ beta ^ {{{-}}}} \ _ {{\ 54}} ^ {{133}} Xe \ {\ xrightarrow [{{5,243} \ {\ d}}] {\ beta ^ {{-}}}} \ _ {{\ 55}} ^ {{133}} Cs}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/26783f606730f3f8a3d33349a46375b70c77d8f5)
![{\ mathrm {^ {{101}} _ {{\ 40}} Zr \ {\ xrightarrow [{{2,3} \ {\ s}}] {\ beta ^ {{-}}}} \ _ { {\ 41}} ^ {{101}} Nb \ {\ xrightarrow [{{7.1} \ {\ s}}] {\ beta ^ {{-}}}} \ _ {{\ 42}} ^ {{101}} Mon \ {\ xrightarrow [{{14.61} \ {\ min}}] {\ beta ^ {{-}}}} \ _ {{\ 43}} ^ {{101}} Tc \ {\ xrightarrow [{{14.22} \ {\ min}}] {\ beta ^ {{-}}}} \ _ {{\ 44}} ^ {{101}} Ru}}](https://wikimedia.org/api/rest_v1/media/math/render/svg/af581afd72cdf3baa6f7391e7f223524d6886216)