Cesium
| properties | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| General | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Name , symbol , atomic number | Cesium, Cs, 55 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Element category | Alkali metals | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Group , period , block | 1 , 6 , p | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Appearance | shiny silver-white, golden-yellow with low traces of oxygen | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS number | 7440-46-2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EC number | 231-155-4 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ECHA InfoCard | 100.028.323 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mass fraction of the earth's envelope | 6.5 ppm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic mass | 132.90545196 (6) et al | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic radius (calculated) | 265 (298) pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Covalent radius | 244 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Van der Waals radius | 343 pm | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electron configuration | [ Xe ] 6 s 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 1. Ionization energy | 3.893 905 695 (24) eV ≈ 375.7 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2. Ionization energy | 23.15745 (6) eV ≈ 2 234.35 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 3. Ionization energy | 33.195 (4) eV ≈ 3 202.8 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 4. Ionization energy | 43.0 (1.7 eV) ≈ 4 150 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 5. Ionization energy | 56.0 (1.9) eV ≈ 5 400kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Physical state | firmly | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
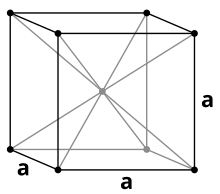
| Crystal structure | body-centered cubic | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| density | 1.90 g / cm 3 (20 ° C ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Mohs hardness | 0.2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| magnetism | paramagnetic ( Χ m = 5.2 10 −6 ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Melting point | 301.59 K (28.44 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| boiling point | 963.2 K (690 ° C) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Molar volume | 70.94 10 −6 m 3 mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of evaporation | 66.1 kJ / mol | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Heat of fusion | 2.09 kJ mol −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Work function | 2.14 eV | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electric conductivity | 4.76 · 10 6 A · V −1 · m −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Thermal conductivity | 36 W m −1 K −1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Chemically | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Oxidation states | +1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Normal potential | −2.923 V (Cs + + e - → Cs) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Electronegativity | 0.79 ( Pauling scale ) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
| For other isotopes see list of isotopes | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
| safety instructions | |||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||
cesium (according to IUPAC ) [ ˈʦɛːzi̯ʊm ] , in standard language cesium or cesium (in American English cesium ), is a chemical element with the element symbol Cs and the atomic number 55. In the periodic table it is in the 1st main group or the 1st IUPAC group and belongs to the alkali metals . Cesium is the heaviest stable alkali metal.
Cesium was discovered in 1861 by Robert Wilhelm Bunsen and Gustav Robert Kirchhoff in the Dürkheim mineral water of the Maxquelle . Because of the two blue spectral lines with which the element was detected, they named it after the Latin caesius for sky blue. The pure element was first presented in 1881 by Carl Theodor Setterberg .
Cesium is an extremely reactive, very soft, gold-colored metal that shines silvery in its highly pure state. Since it reacts immediately and very violently with air, it is stored in sealed glass ampoules under inert gas .
The biological significance of the non-toxic element is unknown. However, due to its similarity to potassium, it is absorbed in the gastrointestinal tract and, like potassium, is mainly stored in muscle tissue. This is why the radioactive isotope cesium-137 ( 137 Cs), a product of nuclear fission , received special attention when it was released in large quantities into the environment as a result of the Chernobyl disaster on April 26, 1986.
history
Cesium was first described by Gustav Robert Kirchhoff and Robert Wilhelm Bunsen in 1861 . They examined mineral water from Dürkheim and after separating calcium , strontium , magnesium and lithium, they discovered two previously unknown lines in the blue spectral range . From their observations, they concluded that there must be another, previously unknown element in the mineral water examined, which they named cesium , after the Latin caesius for “sky blue”, because of the blue spectral lines .
Bunsen also tried to separate cesium from the other alkali metals in order to research further properties of the element. To do this, he mixed the solution with a platinum chloride solution to precipitate potassium and the newly discovered, heavier alkali metals rubidium and cesium as insoluble hexachloridoplatinate . The potassium could be removed by boiling it several times in a little water. To obtain the pure chlorides, the platinum was reduced to an element with hydrogen , so that the now water-soluble cesium and rubidium chlorides could be leached out. The separation of cesium and rubidium took place using the different solubility of the carbonates in absolute ethanol , in which cesium carbonate is soluble in contrast to the corresponding rubidium compound. Cesium chloride was also used by Bunsen and Kirchhoff for an initial determination of the molar mass of the new element, for which they found the value of 123.35 g / mol.
The two researchers were unable to obtain elemental cesium, because the electrolysis of molten cesium chloride produced a blue compound instead of the metal, which they called subchloride , but which was probably a colloidal mixture of cesium and cesium chloride. During the electrolysis of an aqueous solution with a mercury anode , the easily decomposable cesium amalgam was formed .
The presentation of the elemental cesium finally succeeded in 1881 Carl Theodor Setterberg , who avoided the problems with the chloride by the fused salt electrolysis Caesiumcyanid used. The relatively high temperature required to melt the cesium cyanide initially interfered with, but he was able to reduce this temperature using the eutectic with barium cyanide .
Occurrence
With a content of 3 ppm in the continental crust , cesium is a rare element on earth. It is the rarest alkali metal after the unstable francium . Due to its high reactivity, it does not occur elementally, but only in the form of compounds. Usually cesium is a rare accompanying element in potassium or other alkali metal salts such as lepidolite , but some cesium minerals are also known. The most common Caesiummineral is pollucite (Cs, Na) 2 Al 2 Si 4 O 12 · H 2 O, which, in greater numbers, especially on Bernic Lake near Lac du Bonnet in the Canadian province of Manitoba in the Tanco Mine occurs . Other larger deposits are in Bikita , Zimbabwe and Namibia . The deposits in the Tanco mine near Lac du Bonnet are the only ones where cesium is mined. Rarer Caesiumminerale are, for example Cesstibtantit (Cs, Na) SBTA 4 O 12 and Pautovit CSFE 2 S 3 .
Due to the water solubility of most cesium compounds, the element is dissolved in sea water ; one liter contains an average of 0.3 to 4 micrograms of cesium. There are also more common but less soluble elements such as nickel , chromium or copper in comparable quantities .
Extraction and presentation
Cesium is only produced on a small scale. In 1978 the amount of cesium and cesium compounds produced worldwide was about 20 tons. The starting material for the extraction of elemental cesium and all cesium compounds is pollucite , which can be digested with acids or bases. Hydrochloric , sulfuric or hydrobromic acid can be used as acids . This results in a solution containing cesium and aluminum, from which the pure cesium salts are obtained through precipitation , ion exchange or extraction . Another possibility is to heat pollucite with calcium or sodium carbonate and the corresponding chlorides and then leach it out with water . This creates an impure cesium chloride solution.
Cesium metal can be obtained chemically by reducing cesium halides with calcium or barium . In this distillation , the volatile in vacuo Caesiummetall from.
- Reduction of cesium chloride with calcium
Further possibilities of cesium metal production are the reduction of cesium hydroxide with magnesium and the reduction of cesium dichromate with zirconium .
- Reaction of cesium dichromate and zirconium to form cesium, zirconium (IV) oxide and chromium (III) oxide
Highly pure cesium can be produced by the decomposition of cesium azide , which can be obtained from cesium carbonate , and subsequent distillation. The reaction takes place at 380 ° C over an iron or copper catalyst .
properties
Physical Properties

In its purest state, cesium is a silver-white light metal with a density of 1.873 g / cm 3 , which appears golden-yellow due to the slightest contamination. In many properties it stands between those of the rubidium and - as far as is known - those of the unstable francium . At 28.7 ° C, with the exception of francium, it has the lowest melting point of all alkali metals and at the same time has one of the lowest melting points for metals after mercury and comparable to gallium . Cesium is very soft (Mohs hardness: 0.2) and very elastic.
Like the other alkali metals, cesium crystallizes under standard conditions in the cubic crystal system with a body-centered cubic unit cell in the space group Im 3 m (space group no. 229) with the lattice parameter a = 614 pm and two formula units per unit cell. A phase transformation into a face-centered cubic crystal structure with the lattice parameter a = 598 pm takes place under a pressure of 41 kbar .
With the exception of lithium , cesium can be mixed with other alkali metals as desired. With a ratio of 41% cesium, 12% sodium and 47% potassium, an alloy is created with the lowest known melting point of −78 ° C.
The cesium atom and also the ion Cs + have a large radius , they are - again with the exception of francium - the largest individual atoms or ions. This is related to the particularly low effective nuclear charge , which means that the outermost s-electron is only bound to the nucleus to a small extent. In addition to the large atomic radius, this also causes the low ionization energy of the cesium atom and thus the high reactivity of the element.
Gaseous cesium has an unusual index of refraction less than one. This means that the group speed of the electromagnetic wave - in this case light - is greater than in a vacuum , which does not contradict the theory of relativity.
Chemical properties
Cesium is the element with the lowest ionization energy . It has the lowest electronegativity for splitting off the outermost electron . Cesium releases this very easily when it comes into contact with other elements and forms monovalent cesium salts. Since the noble gas configuration is achieved by splitting off this one electron , it does not form any divalent or higher valued ions.
Reactions with cesium are usually very violent, so it ignites immediately on contact with oxygen and, like potassium and rubidium, forms the corresponding hyperoxide .
It also reacts violently with water to form cesium hydroxide ; this reaction even takes place with ice at temperatures of −116 ° C.
When heated with gold , cesium auride (CsAu) is formed, a compound that - despite being formed from two metals - is not an alloy, but a semiconductor; in liquid CsAu there are Cs + and Au - ions.
Isotopes
A total of 41 isotopes and 29 other core isomers of cesium are known. Only the isotope 133 Cs occurs in nature . Cesium is therefore a pure element . Of the artificial isotopes, 134 Cs with 2.0652 years, 135 Cs with 2.33 million years and 137 Cs with 30.08 years have medium to very long half-lives, while those of the other isotopes between 1 µs at 111 Cs and 13.16 Days are 136 Cs.
An important artificial isotope is 137 Cs, a beta emitter with a half-life of 30.08 years. 137 Cs first decays into the metastable intermediate product 137m Ba with a probability of 94.6% , which is converted into the stable barium isotope 137 Ba with a half-life of 2.552 minutes by gamma decay (cf. cesium-barium generator ). In the remaining 5.4% there is a direct transition to the stable barium isotope 137 Ba. Together with other cesium isotopes, it is created either directly during nuclear fission in nuclear reactors or through the decay of other short-lived fission products such as 137 I or 137 Xe.
- Formation of 137 Cs in the nuclear fission of 235 U
In addition to the cobalt isotope 60 Co, 137 Cs is an important source of gamma rays and is used in radiation therapy for the treatment of cancer , for measuring the flow rate in tubes and for checking the thickness of paper, films or metal. In addition, it is used in quality control in nuclear medicine as a long-life nuclide in test sources .
Larger amounts of the isotope 137 Cs were released into the environment through above-ground nuclear weapon tests and through the reactor accidents at Chernobyl and Fukushima . The activity of 137 Cs released in all above-ground nuclear weapons tests was 9.48 · 10 17 Bq . The total amount of 137 Cs released by the Chernobyl disaster had an activity of about 8.5 · 10 16 Bq. In addition, there was an activity of about 4.7 · 10 16 Bq from 134 Cs and 3.6 · 10 16 Bq from 136 Cs. Due to the fallout , many areas in Europe, including Germany, were contaminated with radioactive cesium. 137 Cs is particularly concentrated in fungi , which can decompose lignin and thus have easier access to potassium and thus also to the chemically very similar cesium than plants. In particular, the chestnut boletus ( Boletus badius ) and the flaky-stemmed witch boletus ( Boletus erythropus ) enrich cesium, while the related boletus ( Boletus edulis ) , for example, only shows a low concentration of cesium. The cause of the high concentration of cesium in the first two fungi is due to their hat dyes badion A and norbadion A , which can complex cesium. These two derivatives of pulvic acid are not present in the boletus . Wild animals that eat mushrooms are also affected. The exact cesium load depends on the amount of fallout that has fallen and the nature of the soil, since soils bind cesium to different degrees and thus make it available to plants.
One incident in which people died from radiation exposure to 137 Cs was the Goiânia accident in Brazil in 1987, in which two garbage collectors stole a metal container from an abandoned radiation clinic. The 137 Cs contained in it was distributed to friends and acquaintances because of its striking fluorescent color.
use
Due to the complicated production and high reactivity, elemental cesium is only used to a small extent. It is mainly used in research. Since Cesium a small work function has, it can be as hot cathode those used to obtain free electrons. Also magneto hydrodynamic generators are with cesium as a possible plasma material studied. In space travel, cesium is used as a propulsion means in ion propulsion in addition to mercury and xenon due to its high molar mass, which causes greater recoil than lighter elements .
The second as a unit of measurement of time has been defined since 1967 via the frequency of a certain atomic transition in the cesium isotope 133 Cs. In addition, cesium is the frequency-determining element in the atomic clocks , which form the basis for coordinated universal time . The choice fell on cesium because this is a pure element and in the 1960s the transition between the two basic states with approx. 9 GHz was already detectable with the electronic means of that time. The width of this transition and thus the uncertainty of the measurement is not determined by the properties of the atom. Due to the low evaporation temperature, an atomic beam with low velocity uncertainty can be generated with little effort .
A cloud of cesium atoms can be kept in suspension in magneto-optical traps and cooled down to absolute zero with the help of lasers down to a few microkelvin . With this technology it was possible to significantly improve the frequency stability and thus the accuracy of the cesium atomic clock.
In addition, cesium is used in vacuum tubes because it reacts with small residual traces of gases and thus ensures a better vacuum ( getter ). The cesium is generated in situ by the reaction of cesium dichromate with zirconium . Cesium is - alloyed with antimony and other alkali metals - a material for photocathodes , which are used in photomultipliers, for example.
proof
The spectral lines at 455 and 459 nm in the blue can be used to detect cesium . This can be used quantitatively in flame photometry to determine traces of cesium.
In polarography , cesium shows a reversible cathodic step at −2.09 V (against a calomel electrode ). Quaternary ammonium compounds (for example tetramethylammonium hydroxide ) must be used as the base electrolyte , since other alkali or alkaline earth metal ions have very similar half-wave potentials.
Both cesium and potassium can be detected gravimetrically using various sparingly soluble salts. Examples are the perchlorate CsClO 4 and the hexachloridoplatinate Cs 2 [PtCl 6 ].
Biological importance
Cesium ingested with food is absorbed in the gastrointestinal tract due to its similarity to potassium and, like potassium, is mainly stored in muscle tissue . The biological half-life with which cesium is excreted by the human body depends on age and gender and is 110 days on average.
Cesium is only chemically toxic to a very low degree . Typical LD 50 values for cesium salts are 1000 mg / kg (rat, oral). However, of concern is the effect of ionizing radiation recorded radioactive Caesiumisotope, depending on the dose , the radiation sickness can cause. Due to the good solubility of most cesium salts in water, they are completely absorbed in the gastrointestinal tract and mainly distributed in the muscle tissue. The uptake of radioactive 137 Cs after the Chernobyl disaster in 1986 resulted in an average effective dose of 0.6 μSv for an adult in the Federal Republic of Germany in the first three months .
safety instructions
Cesium ignites spontaneously in air , which is why it must be stored in ampoules under pure argon or in a vacuum . Because of its high reactivity, it reacts explosively with water. The explosiveness can be increased by igniting the hydrogen produced. Burning cesium must be extinguished with metal fire extinguishers or dry sand. As with other alkali metals, they are disposed of by carefully adding dropwise alcohols such as 2-pentanol , tert- butanol or octanol and then neutralizing them .
links
As a typical alkali metal, cesium occurs exclusively in ionic compounds in the +1 oxidation state. Most cesium compounds are readily soluble in water.
Halides
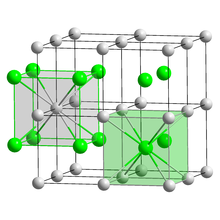
With all halogens, cesium forms easily water-soluble halides of the form CsX (X = halide). Cesium chloride has a characteristic crystal structure that forms an important type of structure (cesium chloride structure). With the exception of cesium fluoride , the other cesium halides also crystallize . Cesium chloride is the starting material for the extraction of elemental cesium. Since a density gradient is formed automatically if the centrifugation is long enough , it is used to separate and purify DNA in the ultracentrifuge . Highly pure cesium iodide and cesium bromide are used as transparent scintillation material in scintillation counters .
Oxygen compounds
Cesium forms an unusually large number of oxygen compounds . This is mainly due to the low reactivity of the cesium ion, so that the formation of oxygen-oxygen bonds is possible. Several suboxides are known, such as Cs 11 O 3 and Cs 3 O, in which there is an excess of cesium and which accordingly exhibit electrical conductivity . In addition, the oxide Cs 2 O, the peroxide Cs 2 O 2 , the hyperoxide CsO 2 and the ozonide CsO 3 are known with increasing oxygen contents . In contrast to most of the other cesium compounds, all these compounds are colored, the suboxides purple or blue-green, the others yellow, orange or red.
Cesium hydroxide is a strongly hygroscopic , white solid that dissolves well in water. Cesium hydroxide is a strong base in aqueous solution .
Other cesium compounds
Cesium carbonate is a white solid and dissolves in many organic solvents. It is used in various organic syntheses as a base, for example for esterifications or for splitting off special protective groups.
Cesium nitrate is widely used in military pyrotechnics , in NIR - flares and Infrarottarnnebeln , while the use in NIR flares to the intense emission lines of the element at 852, 1359 and 1469 nm is based, the use is based in Tarnnebeln on the light ionizability of the element. The Cs ions formed in the flame when the pyrotechnic active compounds burn off act as condensation nuclei and therefore increase the aerosol yield which is important for radiation absorption.
Cesium chromate can be used together with zirconium as a simple source for the extraction of elemental cesium to remove traces of water and oxygen in vacuum tubes.
The category: Cesium compounds gives an overview of cesium compounds .
literature
- Manfred Bick, Horst Prinz: Cesium and Cesium Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry , Wiley-VCH, Weinheim 2005 ( doi: 10.1002 / 14356007.a06_153 ).
- AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 .
- Entry to Cesium. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
Web links
Individual evidence
- ^ Harry H. Binder: Lexicon of the chemical elements. S. Hirzel Verlag, Stuttgart 1999, ISBN 3-7776-0736-3 .
- ↑ The values for the properties (info box) are taken from www.webelements.com (cesium) , unless otherwise stated .
- ↑ CIAAW, Standard Atomic Weights Revised 2013 .
- ↑ Manjeera Mantina, Adam C. Chamberlin, Rosendo Valero, Christopher J. Cramer, Donald G. Truhlar: Consistent van der Waals Radii for the Whole Main Group. In: J. Phys. Chem. A . 113, 2009, pp. 5806-5812, doi: 10.1021 / jp8111556 .
- ↑ a b c d e Entry on cesium in Kramida, A., Ralchenko, Yu., Reader, J. and NIST ASD Team (2019): NIST Atomic Spectra Database (ver. 5.7.1) . Ed .: NIST , Gaithersburg, MD. doi : 10.18434 / T4W30F ( https://physics.nist.gov/asd ). Retrieved June 11, 2020.
- ↑ a b c d e entry on cesium at WebElements, https://www.webelements.com , accessed on June 11, 2020.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the elements. 1st edition. VCH, Weinheim 1988, ISBN 3-527-26169-9 , p. 97.
- ↑ Robert C. Weast (Ed.): CRC Handbook of Chemistry and Physics . CRC (Chemical Rubber Publishing Company), Boca Raton 1990, ISBN 0-8493-0470-9 , pp. E-129 to E-145. Values there are based on g / mol and given in cgs units. The value specified here is the SI value calculated from it, without a unit of measure.
- ↑ a b Yiming Zhang, Julian RG Evans, Shoufeng Yang: Corrected Values for Boiling Points and Enthalpies of Vaporization of Elements in Handbooks. In: Journal of Chemical & Engineering Data . 56, 2011, pp. 328-337, doi: 10.1021 / je1011086 .
- ↑ Ludwig Bergmann, Clemens Schaefer, Rainer Kassing: Textbook of Experimental Physics . Volume 6: Solids. 2nd Edition. Walter de Gruyter, 2005, ISBN 3-11-017485-5 , p. 361.
- ↑ a b c Entry on cesium in the GESTIS substance database of the IFA , accessed on April 30, 2017(JavaScript required) .
- ↑ a b c G. Kirchhoff, R. Bunsen: Chemical analysis through spectral observations. In: Annals of Physics and Chemistry . 189, 7, 1861, pp. 337-381, doi: 10.1002 / andp.18611890702 .
- ↑ Richard Zsigmondy: Colloids and the Ultra Microscope . Read books, 2007, ISBN 978-1-4067-5938-9 , p. 69 ( Colloids and the Ultramicroscope in the Google book search).
- ↑ Carl Setterberg: About the representation of rubidium and cesium compounds and about the extraction of the metals themselves. In: Justus Liebigs Annalen der Chemie . 221, 1, 1881, pp. 100-116, doi: 10.1002 / jlac.18822110105 .
- ↑ a b David R. Lide (Ed.): CRC Handbook of Chemistry and Physics . 90th edition. (Internet version: 2010), CRC Press / Taylor and Francis, Boca Raton, FL, Geophysics, Astronomy, and Acoustics; Abundance of Elements in the Earth's Crust and in the Sea, pp. 14-18.
- ↑ US Geological Survey : Cesium . (PDF file; 82 kB) In: Mineral Commodity Summaries. January 2009.
- ↑ a b c d e f Manfred Bick, Horst Prinz: Cesium and Cesium Compounds. In: Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH , Weinheim 2005 ( doi: 10.1002 / 14356007.a06_153 ).
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1273.
- ^ Fritz Blatter, Ernst Schuhmacher: Production of high purity cesium. In: Journal of the Less Common Metals . 115, 2, 1986, pp. 307-313, doi: 10.1016 / 0022-5088 (86) 90153-0 .
- ↑ K. Schubert: A model for the crystal structures of the chemical elements. In: Acta Crystallographica . B30, 1974, pp. 193-204, doi: 10.1107 / S0567740874002469 .
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1274.
- ↑ Michael Binnewies, Michael Jäckel, Helge Willner: Allgemeine und Anorganische Chemie . Spektrum Akademischer Verlag, Berlin 2003, ISBN 3-8274-0208-5 , pp. 49-53.
- ↑ LJ Wang, A. Kuzmich, A. Dogariu: Gain-assisted superluminal light propagation. In: Nature . 406, 2000, pp. 277-279, doi: 10.1038 / 35018520 .
- ↑ cesium at webelements.com, consulted on 5 September, 2009.
- ↑ Entry on gold. In: Römpp Online . Georg Thieme Verlag, accessed on April 21, 2015.
- ↑ a b G. Audi, FG Kondev, Meng Wang, WJ Huang, S. Naimi: The NUBASE2016 evaluation of nuclear properties. In: Chinese Physics C. 41, 2017, S. 030001, doi: 10.1088 / 1674-1137 / 41/3/030001 ( full text ).
- ↑ Martin Volkmer: Basic knowledge of nuclear energy . Nuclear Energy Information Circle , Bonn 1996, ISBN 3-925986-09-X , p. 30.
- ↑ Radioisotopic letter: Cesium-137 (Cs-137) . Centers for Disease Control and Prevention. Atlanta 2006, accessed September 25, 2009.
- ↑ L. Geworski, Chr. Reiners. Quality testing of nuclear medicine measuring systems: constancy test. In: L. Geworski, G. Lottes, Chr. Reiners, O. Schober . Recommendations for quality control in nuclear medicine. Schattauer Verlag, Stuttgart / New York 2003, ISBN 3-7945-2242-7 , p. 258 and p. 263.
- ↑ a b c UNSCEAR 2008 Report. Sources and effects of ionizing radiation . Volume 2. Annex D - Health effects due to radiation from the Chernobyl accident. New York 2011, p. 49, (PDF)
- ↑ DC Aumann, G. Clooth, B. Steffan, W. Steglich: Complexation of Cesium-137 by the cap dyes of the chestnut boletus (Xerocomus badius). In: Angewandte Chemie . Volume 101, Number 4, 1989, pp. 495-496, doi: 10.1002 / ange.19891010429 .
- ↑ P. Kuad, R. Schurhammer, C. Maechling, C. Antheaume, C. Mioskowski, G. Wipff, B. Spiess: Complexation of Cs +, K + and Na + by norbadione A triggered by the release of a strong hydrogen bond: nature and stability of the complexes. In: Phys Chem Chem Phys. 11, 2009, pp. 10299-10310, doi: 10.1039 / B912518C .
- ↑ B. Steffan, W. Steglich: The hat dyes of the chestnut boletus (Xerocomus badius). In: Angewandte Chemie . Volume 96, Number 6, June 1984, pp. 435-437, doi: 10.1002 / anie.19840960619 .
- ↑ State Institute for the Environment, Measurements and Nature Conservation Baden-Württemberg: Radioactivity pollution in game. ( Memento from June 19, 2008 in the Internet Archive ) 2007.
- ↑ a b c d Entry on Cesium 137, Cesium 134. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.
- ^ IAEA : Radiological Accident in Goiânia. Vienna 1988, HTML (there PDF) , accessed on December 13, 2007.
- ↑ European Space Agency : Ion Thrusters: The Ride on Charged Particles . As of September 2003, accessed on September 26, 2009.
- ^ Bureau International des Poids et Mesures: Resolution 1 of the 13th CGPM (1967). Retrieved April 2, 2019 .
- ↑ Andreas Bauch: Suppliers of the time. In: Physics in Our Time . 25, 4, 1994, pp. 188-198, doi: 10.1002 / piuz.19940250412 .
- ↑ Andreas Bauch: Time measurement with fountains: atomic clocks. In: Physics in Our Time . 32, 6, 2001, pp. 268-273, doi : 10.1002 / 1521-3943 (200111) 32: 6 <268 :: AID-PIUZ268> 3.0.CO; 2-N .
- ↑ Norbert Schaetti: Influencing the characteristics of a Cs-Sb photocathode by adding foreign elements. In: Journal for Applied Mathematics and Physics (ZAMP). 4, 5, 1953, pp. 450-459, doi: 10.1007 / BF02067902 .
- ↑ J. Heyrovský, J. Kuta: Basics of polarography. Akademie-Verlag, Berlin 1965, p. 509.
- ↑ Cesium. In: Lexicon of Chemistry. Spektrum Verlag, Heidelberg 2000.
- ↑ C. Zink et al.: Schering Lexikon Radiologie. 3. Edition. Abw Wissenschaftsverlag, ISBN 3-936072-20-5 , p. 103.
- ↑ Science online lexica: Entry on cesium compounds in the lexicon of chemistry. Retrieved November 14, 2009.
- ↑ Timo Flessner, Sven Doye: Cesium carbonate: A powerful inorganic base in organic synthesis. In: Journal for practical chemistry . 341, 2, 1999, pp. 186-190, doi : 10.1002 / (SICI) 1521-3897 (199902) 341: 2 <186 :: AID-PRAC186> 3.0.CO; 2-6 .
- ↑ E.-C. Koch: Special Materials in Pyrotechnics, Part II: Application of Cesium and Rubidium Compounds in Pyrotechnics. In: J. Pyrotech. 15, 2002, pp. 9-24 ( abstract ( memento of July 13, 2011 in the Internet Archive )).
- ^ CW Lohkamp: USP 3 733 223, The United States as represented by the Secretary of the Navy, USA, (1973).
- ↑ M. Weber DE 32 38 444, Pyrotechnische Fabrik F. Feistel GmbH & Co. KG, Göllheim, (1982).
- ↑ Entry on cesium compounds. In: Römpp Online . Georg Thieme Verlag, accessed on June 19, 2014.