Platinum (IV) chloride
| Crystal structure | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
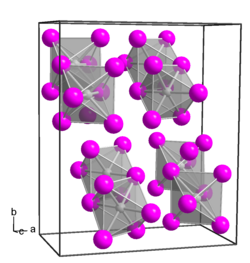
|
|||||||||||||||||||
| __ Pt 4+ __ Cl - | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | Platinum (IV) chloride | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Ratio formula | PtCl 4 | ||||||||||||||||||
| Brief description |
red-brown solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 336.90 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| density |
|
||||||||||||||||||
| Melting point |
Decomposes at 370 ° C |
||||||||||||||||||
| solubility |
good in water (587 g l −1 at 25 ° C) |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| Toxicological data | |||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Platinum (IV) chloride is an inorganic chemical compound of platinum from the group of chlorides .
Extraction and presentation
Platinum (IV) chloride is formed when platinum is dissolved in aqua regia by the decomposition of the hexachloridoplatinic acid which is initially formed ; the deep, but purely yellow solution gives a red-brown salt mass when it evaporates, which turns brown-red when the crystal water is expelled.
It can also be obtained by chlorinating platinum with sulfuryl chloride in vacuo at 350 ° C.
properties
Platinum (IV) chloride is a red-brown, crystalline and hygroscopic salt. It tastes badly sharp, metallic, is soluble in water , ethanol and diethyl ether , colors organic substances brownish-red, gives greenish-gray, insoluble platinum (II) chloride PtCl 2 when heated and ultimately leaves behind platinum .
It is hygroscopic and absorbs water up to the pale yellow pentahydrate PtCl 4 · 5H 2 O on standing in air .
Platinum (IV) chloride has an orthorhombic crystal structure with the space group Pbca (space group no. 61) .
Individual evidence
- ↑ a b c d Georg Brauer (Ed.) U. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume III, Ferdinand Enke, Stuttgart 1981, ISBN 3-432-87823-0 , p. 1709.
- ↑ a b c d e f Entry on platinum (IV) chloride in the GESTIS substance database of the IFA , accessed on July 23, 2016(JavaScript required) .
- ^ A b Jean d'Ans, Ellen Lax, Roger Blachnik: Pocket book for chemists and physicists . Springer DE, 1998, ISBN 3-642-58842-5 , pp. 676 ( limited preview in Google Book search).
literature
- Cotton, SA Chemistry of Precious Metals , Chapman and Hall (London): 1997. ISBN 0-7514-0413-6 .



