Cesium peroxide
| Crystal structure | |||||||
|---|---|---|---|---|---|---|---|
|
|||||||
| General | |||||||
| Surname | Cesium peroxide | ||||||
| other names |
|
||||||
| Ratio formula | Cs 2 O 2 | ||||||
| Brief description |
colorless solid |
||||||
| External identifiers / databases | |||||||
|
|||||||
| properties | |||||||
| Molar mass | 297.8 g mol −1 | ||||||
| Physical state |
firmly |
||||||
| density |
4.74 g cm −3 |
||||||
| Melting point |
590 ° C |
||||||
| safety instructions | |||||||
|
|||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||
Cesium peroxide is an inorganic chemical compound of cesium from the group of peroxides .
Extraction and presentation
Cesium peroxide can be obtained by rapid oxidation with oxygen of cesium dissolved in liquid ammonia at −50 ° C.
It can also be obtained by thermal decomposition of cesium superoxide .
properties
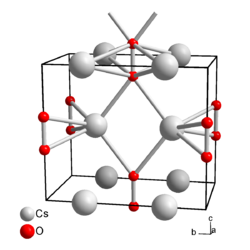
Cesium peroxide is in its purest form a colorless, otherwise yellow, moisture-sensitive, very hard solid that reacts with water to form hydrogen peroxide and cesium hydroxide . It reacts with hydrogen peroxide to form the tetrahydrate. When heated, cesium peroxide decomposes into cesium oxide and oxygen. It has an orthorhombic crystal structure of the space group Immm (space group no. 71) , a = 432.2 pm, b = 751.7 pm, c = 643.0 pm. In the crystal structure, each cesium ion is coordinated by four peroxide ions; each peroxide ion has bonds to eight cesium ions.
use
Cesium peroxide is the active component in silver-oxygen-cesium AgOCs photocathodes .
Individual evidence
- ↑ a b c d e Georg Brauer (Ed.), With the collaboration of Marianne Baudler u. a .: Handbook of Preparative Inorganic Chemistry. 3rd, revised edition. Volume I, Ferdinand Enke, Stuttgart 1975, ISBN 3-432-02328-6 , p. 955.
- ↑ a b c d Jane E. Macintyre: Dictionary of Inorganic Compounds . CRC Press, 1992, ISBN 0-412-30120-2 , pp. 3097 ( limited preview in Google Book Search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ^ RWG Wyckoff, Crystal Structures, 1, 85-237 (1963), Second edition. Interscience Publishers, New York.
