Lithium peroxide
| Crystal structure | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| __ Li + __ O - | ||||||||||||||||
| General | ||||||||||||||||
| Surname | Lithium peroxide | |||||||||||||||
| other names |
Dilithium peroxide |
|||||||||||||||
| Ratio formula | Li 2 O 2 | |||||||||||||||
| Brief description |
white solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 45.88 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
2.36 g cm −3 |
|||||||||||||||
| Melting point |
Decomposition from 340 ° C |
|||||||||||||||
| solubility |
exothermic reaction with water |
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
Lithium peroxide , Li 2 O 2, is an oxygen compound of the alkali metal lithium .
Manufacturing
It is represented by reacting lithium hydroxide with hydrogen peroxide and then heating with elimination of hydrogen peroxide
properties
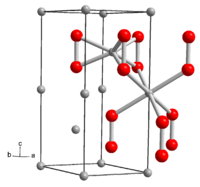
Lithium peroxide is a colorless, usually yellowish solid in its purest form. Hydrogen peroxide is formed with water. The compound has a hexagonal crystal structure with the space group P 6 3 / mmc (space group no. 194) and the lattice parameters a = 3.183 Å and c = 7.726 Å. The crystal structure contains two crystallographically different Li atoms. One is coordinated by the six oxygen atoms of three peroxide ions, the second in a distorted octahedron by the oxygen atoms of six neighboring peroxide ions. The standard enthalpy of formation of lithium peroxide is ΔH f 0 = −633 kJ / mol.
use
Lithium peroxide can be used to produce high-purity lithium oxide . Here, lithium peroxide is decomposed at 195 ° C, with lithium oxide and oxygen being formed:
It is also used in space travel to regenerate life-sustaining gas supply systems. It reacts with carbon dioxide to form lithium carbonate and oxygen. This removes carbon dioxide from the air we breathe and releases oxygen.
Lithium peroxide is used as a hardener for special polymers. It is still used for the lithium peroxide accumulators that are currently being developed or formed in the battery during discharge operation.
Individual evidence
- ↑ Entry on lithium oxide. In: Römpp Online . Georg Thieme Verlag, accessed on July 14, 2014.
- ↑ a b c data sheet lithium peroxide from AlfaAesar, accessed on December 15, 2010 ( PDF )(JavaScript required) .
- ↑ a b data sheet lithium peroxide from Sigma-Aldrich , accessed on April 8, 2011 ( PDF ).
- ^ A b A. F. Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 102nd edition. Walter de Gruyter, Berlin 2007, ISBN 978-3-11-017770-1 , p. 1263.
- ↑ Luis Guillermo Cota, Pablo de la Mora: On the structure of lithium peroxide, Li 2 O 2 . In: Acta Crystallographica Section B Structural Science. 61, 2005, pp. 133-136, doi : 10.1107 / S0108768105003629 .
- ^ AF Holleman , E. Wiberg , N. Wiberg : Textbook of Inorganic Chemistry . 101st edition. Walter de Gruyter, Berlin 1995, ISBN 3-11-012641-9 , p. 1176.
- ^ NN Greenwood, A. Earnshaw: Chemistry of the Elements . 1997, 2nd edition, Oxford: Butterworth-Heinemann, ISBN 0-7506-3365-4 .
- ↑ patent DE2365449 1975 Thiokol Chemical Corp.





