Cesium aurid
| Crystal structure | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
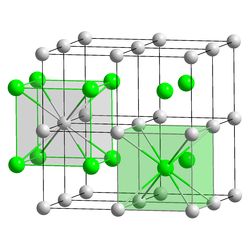
|
|||||||||||||
| __ Cs + __ Au - | |||||||||||||
| Crystal system |
cubic |
||||||||||||
| Space group |
Pm 3 m (No. 221) |
||||||||||||
| General | |||||||||||||
| Surname | Cesium aurid | ||||||||||||
| Ratio formula | CsAu | ||||||||||||
| Brief description |
yellow to red crystals |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 329.87 g · mol -1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| density |
7.065 g cm −3 |
||||||||||||
| Melting point |
590 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Cesium auride (CsAu) is an ionic compound that contains the unusual Au - ion. It was discovered in 1978 in the Joseph Lagowski laboratory . The compound forms when a stoichiometric mixture of cesium and gold is heated ; the two yellow metals give a clear product. The solution in liquid ammonia is brown, while the solid looks yellow; the ammonia adduct is dark blue. Although the compound is formed from two metals, it has no metallic properties and is instead built up ionically. The gold can be deposited on the anode from polar solvents.
The compound reacts violently with water to form cesium hydroxide , metallic gold and hydrogen . In liquid ammonia, the cesium ion can be exchanged for the tetramethylammonium ion using ion exchangers.
Individual evidence
- ↑ a b c W. Freyland, A. Goltzene, P. Grosse, G. Harbeke, H. Lehmann, O. Madelung, W. Richter, C. Schwab, G. Weiser, H. Werheit, W. Zdanowicz: Physics of non-tetrahedrally Bonded elements and Binary compounds I / Physics of non-tetrahedral-linked elements and binary compounds I: . Springer Science & Business Media, 1983, ISBN 3-540-11780-6 , pp. 123 ( limited preview in Google Book search).
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ WJ peer and JJ Lagowski (1978), Metal-Ammonia Solutions. 11. Au - , a solvated transition metal anion in: J. Am. Chem. Soc. 100, 6260-6261.
- ↑ B. Busse, K. Weil: Existence and binding energy of the cesium auride molecule. In: Angewandte Chemie , (1979), doi : 10.1002 / anie.19790910825
- ↑ James E. Huheey, Ellen A. Keiter, Richard L. Keiter: Inorganic Chemistry: Principles of Structure and Reactivity, 4th Edition, Walter de Gruyter 2012, pp. 599f
- ↑ Martin Jansen: Effects of relativistic motion of electrons on the chemistry of gold and platinum . In: Solid State Sciences . 7, No. 12, November 30, 2005, pp. 1464-1474. doi : 10.1016 / j.solidstatesciences.2005.06.015 .