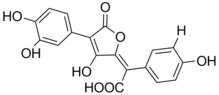
Pulvic acid
| Structural formula | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
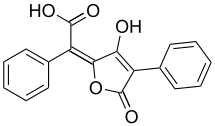
|
||||||||||
| General | ||||||||||
| Surname | Pulvic acid | |||||||||
| other names |
|
|||||||||
| Molecular formula | C 18 H 12 O 5 | |||||||||
| Brief description |
orange prisms |
|||||||||
| External identifiers / databases | ||||||||||
|
||||||||||
| properties | ||||||||||
| Molar mass | 308.29 g mol −1 | |||||||||
| Physical state |
firmly |
|||||||||
| Melting point |
216–217 ° C (decomposition) |
|||||||||
| pK s value |
6.86 ± 0.05 |
|||||||||
| solubility |
|
|||||||||
| safety instructions | ||||||||||
|
||||||||||
| Toxicological data | ||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||
The pulvinic acid is an organic chemical compound , to the lactones , enols and unsaturated carboxylic acids (also alkenoic ) counts. It gave its name to the group of pulvic acid dyes that are widely used in fungi and lichens . Compared to its methyl ester vulpinic acid , pulvic acid has a much lower toxicity.
History and occurrence
There are many different dyes in mushrooms such as the bolete relatives and lichens , which are either derivatives of pulvic acid ( e.g. gomphidic acid ) or composed of several pulvic acid units (example of two units: badiones ). In 1831, the pulvic acid methyl ester vulpinic acid was discovered by the French pharmacist and chemist Antoine Bebert while studying lichens, but it was not until 1860 that Franz Möller and Adolph Strecker discovered and described it in more detail.
Chemical properties
Pulvic acid is a lactone , i.e. an intramolecular ester of trans -1,4-diphenyl-2,3-dihydroxy-1,3-butadiene-1,4-dicarboxylic acid and is formed from this by splitting off water:
The hydroxypulvic acids xerocomic acid and variegate acid, which are found in many of the thick boletus, are the starting compounds for the blue coloration of the fruiting body when it is pressure or injured, as they are oxidized to blue quinone derivatives by enzymes (oxidases) .
Xerocomic acid Variegate acid
presentation
Jacob Volhard was the first to synthesize both pulvic acid and vulpinic acid. By reaction with diethyl oxalate with phenylacetonitrile in a molar ratio of 1: 2, a condensation product is obtained which, after acidic hydrolysis, gives pulvic acid.
Another laboratory synthesis starts from 2,5-diphenyl- p -benzoquinone 1 . This is reacted with bromine in glacial acetic acid to give 3,6-dibromo-2,4-diphenyl- p- benzoquinone 2 . Polyporic acid 4 is obtained from the dibromo compound with a sodium hydroxide solution in methanol at room temperature . Oxidation of the polyporic acid with lead tetraacetate gives the lactone 5 , which after acidic hydrolysis gives the pulvic acid 6 .
biosynthesis
In mushrooms, biosynthesis takes place via tyrosine or phenylalanine ; After deamination, dimerization and ring cleavage, pulvic acid or hydroxypulvic acid is finally produced.
Biological importance
Many yellow pigments in mushrooms and lichens are derived from pulvic acid. A distinction is made between derivatives of pulvic acid and di- and oligomeric substances. The dimers include the badione ; Derivatives of the monomer are, for example, gomphidic acid and vulpinic acid . Vulpinic acid, the methyl ester of pulvic acid, is a strong poison that protects the producing fungi and lichens from snails and other predators.
Individual evidence
- ↑ a b c Entry on pulvic acid. In: Römpp Online . Georg Thieme Verlag, accessed on July 26, 2014.
- ↑ Claudia Synowietz (Ed.): Paperback for chemists and physicists . founded by Jean d'Ans, Ellen Lax. 4th edition. Volume II: Organic Compounds . Springer, Berlin 1983, ISBN 3-540-12263-X .
- ^ MC Gaylord, LR Brady: Comparison of pigments in carpophores and saprophytic cultures of Paxillus panuoides and Paxillus atrotomentosus . In: Journal of Pharmaceutical Sciences . tape 60 , no. 10 , 1971, p. 1503-1508 , doi : 10.1002 / jps.2600601013 .
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ Acta Pharmacologica et Toxicologica , Vol. 2, p. 109, 1946.
- ^ National Academy of Sciences, National Research Council, Chemical-Biological Coordination Center, Review . Vol. 5, p. 0, 1953.
- ↑ Canstatt's Annual Report on Advances in Pharmacy and Allied Sciences in All Countries , Harvard University, Volume 10 (1861).
- ^ A b Tilo Lübken: Hygrophorone. New antifungal cyclopentenone derivatives from Hygrophorus species (Basidiomycetes). (PDF; 3.3 MB) University of Halle, Halle ad Saale 2006. pp. 11–12.
- ↑ J. Volhard: Synthesis and Constitution of Vulpinsäure . In: Justus Liebig's Annals of Chemistry . tape 282 , no. 1-2 , 1894, pp. 1 , doi : 10.1002 / jlac.18942820102 .
- ^ Robert L. Frank, George R. Clark, James N. Coker: The Synthesis of Vulpinic Acid from Polyporic Acid . In: Journal of the American Chemical Society . tape 72 , no. 4 , April 1950, p. 1824 , doi : 10.1021 / ja01160a121 .
- ^ L. Zechmeister: Advances in the chemistry of organic natural substances , 1971, Springer-Verlag , ISBN 3-211-81024-2 .