Polyporic acid
| Structural formula | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
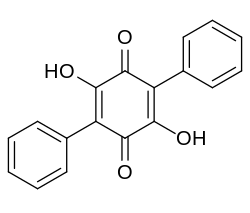
|
|||||||||||||
| General | |||||||||||||
| Surname | Polyporic acid | ||||||||||||
| other names |
|
||||||||||||
| Molecular formula | C 18 H 12 O 4 | ||||||||||||
| Brief description |
bronze colored plates |
||||||||||||
| External identifiers / databases | |||||||||||||
|
|||||||||||||
| properties | |||||||||||||
| Molar mass | 292.29 g mol −1 | ||||||||||||
| Physical state |
firmly |
||||||||||||
| Melting point |
310-312 ° C |
||||||||||||
| safety instructions | |||||||||||||
|
|||||||||||||
| Toxicological data | |||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||
Polyporic acid is a mushroom ingredient and the simplest member of the terphenylquinone class . The connection was first described in 1877 by Carl Stahlschmidt (1831-1902). The structure of polyporic acid was clarified by Fritz Kögl in 1926 .
Occurrence
Polyporic acid occurs in some tree fungi of the order Aphyllophorales and in the leaf lichen Sticta coronata . In the case of the cinnamon-colored soft pork ( Hapalopilus rutilans ) in particular , the dry matter consists of up to 43.5% (according to other information about 20%) of polyporic acid.
properties
Polyporic acid forms red solvates from pyridine and yellow solvates from dioxane . In dilute alkalis, the compound dissolves as a dye with a deep violet color and precipitates as an ocher-yellow solid when acidified. The deep purple potassium salt crystallizes with concentrated potassium hydroxide solution .
Polyporic acid is responsible for symptoms of poisoning that occur after consuming the cinnamon-colored soft porcine with a latency period of around 12 hours. These are characterized by central nervous disorders ( visual and coordination disorders ) and kidney failure . One symptom of polyporic acid poisoning - also known as polyporic acid syndrome - is the purple color of the urine.
The screening of plant products showed that a preparation made from lichen containing polyporic acid significantly extended the lifespan of mice that had previously been vaccinated with acute lymphoblastic leukemia . Polyporic acid was identified as the active component. Synthetic polyporic acid showed a similar antileukemic effect as the natural preparation. So far, the antileukemic effect of polyporic acid, which was reported in 1959, or any chemical derivatives derived from it, has not found any medical application.
Polyporic acid and various of its 3,6-derivatives reduce the vegetative reproduction and spore germination of twelve types of fungus belonging to six different genera .
biosynthesis
Polyporic acid is produced during biosynthesis through the dimerization of phenylpyruvic acid .
Laboratory syntheses
In the Friedrich Fichter synthesis route , 1,4-diphenylbutane-2,3-dione 1 is reacted with diethyl oxalate 2 in the presence of sodium in the sense of a double keto-Claisen condensation . From the primarily resulting cyclohexantetron derivative 3 , the polyporic acid 4 is obtained via a keto-enol tautomerism .
Another laboratory synthesis with better yields starts from p -benzoquinone 1 . According to a method published by Rudolf Pummerer , this is reacted with benzene in the presence of AlCl 3 to form 2,5-diphenyl- p -benzoquinone 2 . The diphenylbenzoquinone is reacted with bromine in glacial acetic acid to give 3,6-dibromo-2,4-diphenyl- P -benzoquinone. Polyporic acid 4 is obtained from the dibromo compound with a sodium hydroxide solution in methanol at room temperature .
Individual evidence
- ↑ a b c d e Entry on polyporic acid. In: Römpp Online . Georg Thieme Verlag, accessed on September 4, 2019.
- ↑ This substance has either not yet been classified with regard to its hazardousness or a reliable and citable source has not yet been found.
- ↑ a b c J. Langner, S. Bauer, J. Miersch, F.-W. Rath: On the biological effect of polyporic acid. Toxicological and biochemical processing of two cases of poisoning with the cinnamon-colored soft pork (Hapalopilus rutilans) . In: Journal of Mycology . tape 58 , no. 2 , 1992, p. 173-184 ( dgfm-ev.de [PDF]).
- ↑ C. Stahlschmidt: About a new naturally occurring organic acid . In: Justus Liebig's Annals of Chemistry . tape 187 , no. 2-3 , 1877, pp. 177 , doi : 10.1002 / jlac.18771870204 .
- ^ A b Fritz Kögl: Investigations on mushroom dyes. V. The constitution of polyporic acid . In: Justus Liebig's Annals of Chemistry . tape 447 , no. 1 , 1926, pp. 78 , doi : 10.1002 / jlac.19264470108 .
- ↑ Jan-Markus Teuscher: New experimental designs on the subject of natural substances in chemistry classes: Chemistry with mushrooms . Dissertation. Friedrich Schiller University Jena, Jena 2011, DNB 1017067767 , p. 99 ( db-thueringen.de [PDF]).
- ↑ Poisoning Syndromes. Polyporic Acid Syndrome, Hapalopilus Syndrome. German Society for Mycology eV, accessed on September 4, 2019 .
- ↑ JF Burton, BF Cain: Antileukæmic Activity of Polyporic Acid. In: Nature. Volume 184, 1959, pp. 1326-1327, doi: 10.1038 / 1841326a0 .
- ^ M. Gill, W. Steglich : Pigments of Fungi ( Macromycetes ) . In: Progress in the Chemistry of Organic Natural Products . Springer Science & Business Media, 1987, ISBN 3-7091-6971-2 , chap. 2.1.2 Polyporic acid and derivatives, p. 18 (English).
- ^ D. Brewer, WSG Maass, A. Taylor: The effect on fungal growth of some 2,5-dihydroxy-1,4-benzoquinones. In: Canadian Journal of Microbiology. Volume 23, 1977, pp. 845-851, doi: 10.1139 / m77-126 .
- ↑ Fr. Fichter: About synthetic p-dialkylated dioxyquinones . In: Justus Liebig's Annals of Chemistry . tape 361 , no. 2-3 , 1908, pp. 363 , doi : 10.1002 / jlac.19083610209 .
- ^ The Complete Book on Natural Dyes & Pigments . Asia Pacific Business Press, Delhi 2005, ISBN 81-7833-032-6 , pp. 319 ( limited preview in Google Book search).
- ↑ Rudolf Pummerer, Ernst Prell: About the addition of benzene to quinone . In: Reports of the German Chemical Society . tape 55 , no. 9 , October 14, 1922, p. 3105 , doi : 10.1002 / cber.19220550920 .
- ^ Robert L. Frank, George R. Clark, James N. Coker: The Synthesis of Vulpinic Acid from Polyporic Acid . In: Journal of the American Chemical Society . tape 72 , no. 4 , April 1950, p. 1824 , doi : 10.1021 / ja01160a121 .
- ↑ PR Shildneck, Roger Adams: THE SYNTHESIS OF POLYPORIC ACID AND DIMETHYL ETHER ATROMENTIN . In: Journal of the American Chemical Society . tape 53 , no. June 6 , 1931, p. 2373 , doi : 10.1021 / ja01357a053 .

