Terphenylquinones
Terphenylchinone are mushroom dyes from the group of substances phenyl-substituted p -Benzochinone .
General chemical structure of terphenylquinones
Derivatives with a central o -benzoquinone structural element are also known.
biosynthesis
The biosynthesis of terphenylquinones takes place through the dimerization of substituted phenylpyruvic acids .
Occurrence
The Terphenylchinone are typical ingredients of Dickröhrlingsartigen (Boletales).
Examples
designation structure CAS no. Occurrence Polyporic acid 
548-59-4 Tree fungi of the order Aphyllophorales , leaf lichen Sticta coronata Atromentine 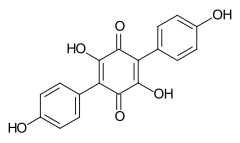
519-67-5 Samtfusskrempling (Paxillus atrotomentosus, Basidiomycetes) Aurantiacin 
548-32-3 Hydnellum aurantiacum (Orange-yellow cork spike, Basidiomycetes ) Phlebial rubron 
7204-23-1 Cultures of Phlebia strigoso-zonata and Punctularia atropurpurascens (Basidiomycetes) Spiromentin B 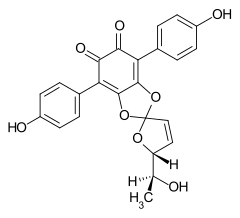
121254-56-6 Velvet foot krempling (Paxillus atrotomentosus, Basidiomycetes) and cultures of Paxillus panuoides
Individual evidence
- ↑ Burkhard Fugmann (Ed.): RÖMPP Lexikon Naturstoffe . 1st edition. Georg Thieme Verlag, Stuttgart 1997, ISBN 3-13-749901-1 , p. 638 ( limited preview in Google Book search).
- ↑ Entry on polyporic acid. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2019.
- ↑ Entry on atromentin. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2019.
- ↑ External identifiers or database links for aurantiacin : CAS number: 548-32-3, PubChem : 261132 , ChemSpider : 229231 , Wikidata : Q83076639 .
- ↑ Entry on aurantiacin. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2019.
- ↑ Entry on Phlebiarubron. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2019.
- ↑ Entry on Spiromentine. In: Römpp Online . Georg Thieme Verlag, accessed on September 3, 2019.
