Tautomerism
The tautomerism (from gr. Tautó "the same" and meros "part") describes a special form of isomerism in chemistry . It was discovered in 1876 by Alexander Michailowitsch Butlerow (1828–1886) and introduced as a term in 1885 by Conrad Peter Laar .
| Overview | |||
|---|---|---|---|
| A-X-Y = Z with A = H + | X = Y-Z-A | Prototropy | |
| H-C-C = O | C = C-O-H | Keto-enol | |
| H-C-N = O | C = N-O-H | Nitro-acinitro | Nitroso oxime |
| H-C-N = N | C = N-N-H | Azo hydrazo | Hydrazo-azo |
| H-N-C = O | N = C-O-H | Amide-imidic acid | Lactam-lactim |
| H-C-C = N | C = C-N-H | Imine-enamine | |
| Ring-chain tautomerism | |||
| A – X – Y = Z with A = OH - | Anionotropy | ||
| HO-C-C = C | C = C-C-OH | ( see below ) | |
| A-X-Y | X-Y-H | dyadic tautomerism | |
When molecules have the same molecular formula but the individual atoms are linked differently, one speaks of constitutional isomers . Tautomers are isomers that quickly merge into one another due to the migration of individual atoms or groups of atoms. H. the two isomers are in dynamic chemical equilibrium with one another. Because of the rapid equilibrium, the individual tautomers can often not be isolated; the ratio of the tautomers to one another is constant.
Tautomers often differ in the position of a group and in the position of a double bond:
Monovalent cations such as the proton or monovalent anions such as chloride , hydroxide or acetate ions come into consideration as migrating groups . If a double bond is replaced by a ring formation from single bonds, one speaks of ring-chain tautomerism .
Tautomerism must not be confused with mesomerism , in which only the same molecule is described by different limit formulas.
Prototropy
In prototropy , a proton changes its place in the molecule:
Keto-enol tautomerism
The most common form of tautomerism is keto-enol tautomerism . Because of the polarization of the C – O double bond due to the high electronegativity of the oxygen and the possibility of delocalizing the negative charge over three atoms after deprotonation , protons at the α-C atom of the carbonyl group can easily be split off. Reprotonation of the enolate ion on oxygen leads to the enol. This tautomerization can be catalyzed by bases, which support the cleavage of the proton, or by acids, which reinforce the polarization of the C – O bond by protonating the carbonyl oxygen.
| Keto-enol tautomerism | |||||
|---|---|---|---|---|---|

|
 
|
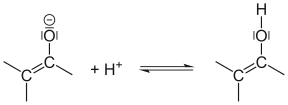
|
|||
| Splitting off a proton: The α-C atom releases a proton in a basic solution. | This carbanion is mesomeric stabilized : Mesomeric stabilization of the enolate anion. | If the proton binds to the negative oxygen, the enol is created: the proton is attached to the enolate anion. | |||
| Carbonyl compound | Enol form in% |
|---|---|
| Propanone ( acetone ) | 0.00025 |
| Butane-2,3-dione ( diacetyl ) | 0.0056 |
| Cyclohexanone | 0.02 |
| Ethyl 3-oxobutyrate ( acetoacetic ester ) | 8th |
| Pentane-2,4-dione ( acetylacetone ) | 80 |
| monohydric phenols | 100 |
The balance is usually on the side of the keto form. The proportion of the enol form in acetone is only 0.00025%. In the case of pentane-2,4-dione (common name acetylacetone ), however, the enol form predominates in equilibrium. Phenols are mainly in the enol form, since the formation of the keto form (example: quinoid structure) abolishes the aromatic system.
Example: Ethanal and ethenol are in a tautomeric equilibrium in solution, but the equilibrium is clearly on the side of the ethanal. Both bond isomerism (C = C / C = O) and functional isomerism (-CH = O / -C-OH) are present.
| Ethanal-Ethenol Tautomerism |
|---|
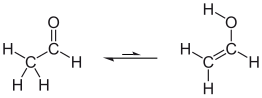
|
The reactivity cannot be inferred from the position of the equilibrium. The enol form is often considerably more reactive than the keto form. However, because this is constantly being reproduced due to the rapidly established equilibrium, only the reactivity of the enol is observed macroscopically (see Le Chatelier's principle ).
This can be shown very nicely in the reaction of acetone with bromine. After adding a drop of bromine, the color of the bromine disappears slowly at first, then faster and faster, since the HBr that is formed greatly accelerates the establishment of the keto-enol equilibrium. If more bromine is added, the discoloration occurs almost instantaneously with the formation of bromoacetone .
Ketol-enediol tautomerism
α- Hydroxy-ketones ( acyloins ) show a special form of keto-enol tautomerism.
1. 2-Hydroxy-propanal has a hydroxyl group on the α-C atom (the 2nd C atom). Due to its negative inductive effect, this polarizes the CH bond on the α-C atom and thus facilitates the splitting off of a proton. An enediol is formed as a molecule with a double bond and two (- di -) adjacent hydroxyl groups. By rearranging a proton, it can be converted back into a molecule with a carbonyl oxygen, in this case a ketone.
| Hydroxypropanal-propenediol-hydroxypropanone tautomerism |
|---|
There is also positional isomerism here, since 2-hydroxypropanal and 1-hydroxypropanone differ only in the positions of the hydroxyl and carbonyl groups.
2. If, in the example of propanal, the methyl group is replaced by a hydrocarbon chain with four carbon atoms and four hydroxyl groups, an aldohexose is present, which is in equilibrium with ketohexose in aqueous solution via the enediol form . Thus, in aqueous solution, the epimeric glucose and mannose are in equilibrium as aldohexoses with the ketohexose fructose ( Lobry-de-Bruyn-Alberda-van-Ekenstein rearrangement ). The previously accepted explanation that the positive Fehling test for fructose can also be traced back to a keto-enol tautomerism has meanwhile been refuted. However, endiols (or their anions, as they are formed in the alkaline Fehling's solution) are themselves strong reducing agents that are dehydrated to 1,2-diketones. For example, benzoin and acetoin (3-hydroxybutanone-2) also have a strong reducing effect . In glycolysis , the conversion of glucose-6-phosphate into fructose-6-phosphate is catalyzed by the enzyme glucose-phosphate isomerase , in a later step of glycolysis the reaction of glyceraldehyde-3-phosphate to dihydroxyacetone phosphate is catalyzed by the enzyme triose phosphate isomerase .
Further examples: ribose and arabinose as aldopentoses are in tautomeric equilibrium with the ketopentose ribulose .
3. Epimerization is also a tautomerization: In aqueous solution, epimers, that is, aldoses that differ only in the position of the hydroxyl group on the 2nd carbon atom, convert into one another. Examples of epimer pairs: glucose / mannose, ribose / arabinose, erythrose / threose , D - glyceraldehyde / L -glyceraldehyde
See also: ketol-enediol tautomerism in phloroglucinol , reductones .
Nitro aci nitro tautomerism
Compounds with a nitro group are in equilibrium with their aci form in acidic solution . The equilibrium is usually on the side of the nitro compound.
| Nitro aci nitro tautomerism |
|---|

|
| Nitroethane : tautomerism of the nitro group |
Nitroso-oxime tautomerism
Compounds with a nitroso group are in equilibrium with their oxime form in acidic solution . The equilibrium is usually 100% on the side of the oxime.
| Nitroso-oxime tautomerism |
|---|

|
Amide-imidic acid tautomerism
The imidic acids are tautomers of the amides .
| Amide-imide tautomerism | ||
|---|---|---|

|

|

|
| Tautomerism amide / imidic acid | Urea tautomerism | Tautomerism of hydroxamic acid |
Imine-enamine tautomerism
| Imine-enamine tautomerism | |
|---|---|
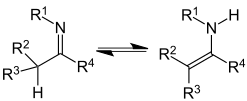
|

|
| Imine- Enamin tautomerism | An example of a cyclic and double imine-enamine tautomerism is histidine . |
Azo-hydrazo-tautomerism
Azo compounds with an enolizable hydrogen atom on the α-carbon atom next to the azo group are in equilibrium with the hydrazo form. As a rule, the equilibrium is predominantly on the side of the hydrazo form.
| Azo-hydrazo-tautomerism |
|---|

|
Tautomerism in tetrazoles
Tautomeric equilibrium of the heteroaromatic 1 H -etrazoles and 2 H -etrazoles in comparison with 5 H -etrazole (right):
| Tetrazole tautomerism |
|---|
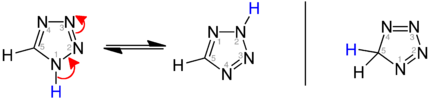
|
Lactam-lactim tautomerism
| Lactam-lactim tautomerism | ||
|---|---|---|

|

|

|
| Tautomerism of a lactam | Cyanuric acid tautomerism | Tautomerism of hypoxanthin (a purine ) |

|
|
Guanine also has an NH 2 group in position 2 , uric acid in positions 2 and 8 each have an OH group |
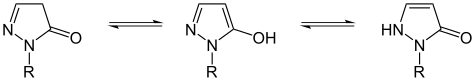
| Tautomerism of barbituric acid (a pyrimidine ) | Tautomerism of dihydropyrazolones | |
Thiolactam-thiolactim tautomerism
| Thiolactam-thiolactim tautomerism |
|---|

|
| Tautomerism of the 6- purinthiol : 6-thioguanine also has an NH 2 group in position 2 |
Ring-chain tautomerism
Oxy-Cyclo-Tautomerism
In the case of long-chain oxo compounds, cyclo- ethers could form. An example is hemiacetal cyclo-tautomerism . Half-acetals are formed in an addition reaction of alcohols with carbonyl compounds. If a molecule contains a hydroxyl group that is far enough away from the carbonyl group, ring closure can occur as a result of a reaction within the molecule. The carbonyl oxygen receives the proton of the hydroxyl group and thus becomes a hydroxyl group itself. The ring closure occurs through the formation of a bond between the negatively polarized oxygen of the removed hydroxyl group and the positively polarized carbon of the carbonyl group. In aqueous solution, there is an equilibrium between the open-chain aldehyde or keto form and the ring form. This is the case with all aldo and keto pentoses and hexoses in aqueous solution.
| Oxy-Cyclo-Tautomerism | |
|---|---|

|

|
| Cyclo-acetal equilibrium | Ribose tautomerism |
(compare ATP and RNA ; see also glucose and fructose )
Tropanol-Cycloheptanone Tautomerism
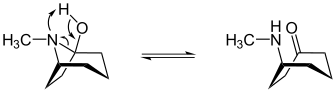
The (1 R ) -1- tropanol is in a tautomeric equilibrium with ( R ) - (methylamino) cycloheptanone:
| Tropanol-heptanone tautomerism |
|---|
|
| Tautomerism of (1 R ) -1-tropanol |
Anionotropy
| Anionotropy |
|---|
| Tautomerism of butenole |
Example of butenols : 3-Hydroxy-1-butene is in tautomeric equilibrium with 1-hydroxy-2-butene ( crotyl alcohol ) if it is heated to 100 ° C. for several hours with dilute sulfuric acid (equilibrium ratio 3: 7). This tautomerism corresponds only formally to an anionotropy in which a hydroxide anion changes its position. The actual reaction mechanism consists in the fact that the hydroxyl group is first protonated and then split off as a water molecule. What remains is a mesomeric-stabilized carbenium ion , which is positively polarized on the 1st and 3rd carbon atom. A water molecule can bind to one of the two carbon atoms, which becomes a hydroxyl group again by splitting off a proton.
Dyadic tautomerism
In dyadic tautomerism (from Greek dyas = two) proton migration takes place between neighboring atoms.
| Dyadic tautomerism | |
|---|---|
|
|
|
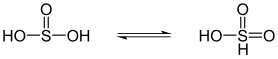
Sulphurous acid is in equilibrium with sulphonic acid |
Hydrogen cyanide ( hydrocyanic acid ) is in equilibrium with hydrogen isocyanide, the equilibrium being on the side of hydrogen cyanide (left). |
Individual evidence
- ↑ Entry on tautomerization . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.T06253 Version: 2.3.3.
- ^ L Antonov: Tautomerism: Methods and Theories , 1st edition, Wiley-VCH, Weinheim 2013, ISBN 978-3-527-33294-6 .
- ↑ a b entry on tautomerism . In: IUPAC Compendium of Chemical Terminology (the “Gold Book”) . doi : 10.1351 / goldbook.T06252 Version: 2.3.3.
- ↑ Holger Fleischer: Misinterpretation of the Fehling's test for reducing sugars - From observation in chemistry class to evidence against the oxidation of the aldehyde group . In: CHEMKON . tape 24 , no. 1 , 2017, ISSN 1521-3730 , p. 27–30 , doi : 10.1002 / ckon.201610283 .
- ↑ Entry on imidic acids. In: Römpp Online . Georg Thieme Verlag, accessed on February 23, 2019.



