Hexoses
Hexoses are monosaccharides with a carbon backbone containing six carbon atoms. They all have the empirical formula C 6 H 12 O 6 and differ in the type of carbonyl functional group. If it is a keto group , one speaks of ketohexoses, in the case of an aldehyde group it is called aldohexoses. The remaining carbon atoms in the structure carry hydroxyl groups . The best-known representatives of the hexoses are glucose (an aldohexose) and fructose (a ketohexose). Furthermore, D - galactose and D - mannose are very important in nature, L - sorbose is of industrial interest for the vitamin C synthesis. In the system of carbohydrates, aldohexoses and ketohexoses are in turn subgroups of aldoses and ketoses .
Aldohexoses
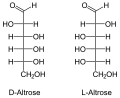
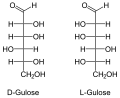
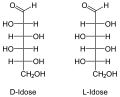
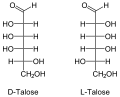
The aldohexoses have four chiral centers , so there are 16 stereoisomers ( ). Only D- glucose, D - galactose and D - mannose occur in nature in larger quantities. D -Talose occurs as a component of the antibiotic hygromycins formed by streptomycetes . In aldohexoses, the D / L configuration always relates to the position of the OH group on the 5th carbon atom and has no relation to the sign [(+) or (-)] of the rotation value α.
All aldohexoses in Fischer projection
The names of the aldohexoses can be easily remembered with the following donkey bridge: "All old geese would like to dance in the garden", whereby the first letters of the proverbs correspond to those of the aldohexoses (optionally also "cluck" instead of "geese", then the risk of confusion is with the Sugars beginning with G lower, “Good” instead of “Glad” refers more closely to Gulose. So: All Old Gluckers Like To Dance Well In The Garden).
Through an intramolecular hemiacetal formation, these open-chain molecules are in equilibrium with the six-membered pyranose or five-membered furanose form . The pyranoses have an oxygen bridge between the 1st and 5th, the furanoses between the 1st and 4th carbon atoms. The oxygen atom of the aldehyde group becomes the hydroxyl group. This creates another center of chirality . The two resulting configurational isomers are called “ anomers ” and are distinguished by the prefix α- and β-.
Ketohexoses
The ketohexoses (synonym hexuloses ) have three chiral centers , so there are eight stereoisomers ( ). The D / L configuration also relates to the position of the OH group on the 5th C atom and has no influence on the direction of the optical activity. Only the four D isomers occur in nature . Another hexulose is fuculose .
All ketohexoses in Fischer projection
See also
Trioses , tetroses , pentoses , heptoses , glucose metabolism
Individual evidence
- ↑ Paula Y. Bruice: Organic Chemistry. 5th updated edition. Pearson Studium, Munich et al. 2007, ISBN 978-3-8273-7190-4 , pp. 1118–1119.