Old rose
| Structural formula | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|

|
|||||||||||||||||||
| Fischer projection , open-chain representation | |||||||||||||||||||
| General | |||||||||||||||||||
| Surname | D - (+) - old rose, L - (-) - old rose | ||||||||||||||||||
| other names |
|
||||||||||||||||||
| Molecular formula | C 6 H 12 O 6 | ||||||||||||||||||
| Brief description |
white solid |
||||||||||||||||||
| External identifiers / databases | |||||||||||||||||||
|
|||||||||||||||||||
| properties | |||||||||||||||||||
| Molar mass | 180.16 g mol −1 | ||||||||||||||||||
| Physical state |
firmly |
||||||||||||||||||
| Melting point |
106-108 ° C |
||||||||||||||||||
| safety instructions | |||||||||||||||||||
|
|||||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | |||||||||||||||||||
Altrose is a monosaccharide with six carbon atoms. This sugar belongs to the group of non-naturally occurring aldohexoses with the molecular formula C 6 H 12 O 6 .
As with any sugar (except for dihydroxyacetone) there are two enantiomeric forms that behave like an image and a mirror image. When "Altrose" is mentioned in this text or in the scientific literature without any additional name ( prefix ), D- Altrose is meant.
properties
In aqueous solution, an intramolecular ring closure sometimes occurs, so that an equilibrium is established between the aldehyde form and the two ring forms ( furanose and pyranose ):
| D -Altrose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
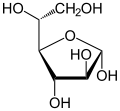 α- D -Altrofuranose 20% |
 β- D -Altrofuranose 13% |
 α- D -Altropyranose 27% |
 β- D -Altropyranose 40% |
|
proof
Fehling's reaction
The detection of the aldehyde group in an aqueous solution of a mixture of copper (II) sulfate (Fehling I) and basic potassium-sodium-tartrate solution (Fehling II) is positive, solid copper (I) oxide (brick-red) is formed Precipitation) (see Fehling's sample ).
Tollens reaction (silver mirror sample)
The Ag + ion in silver nitrate solution is reduced to elemental silver by the old rose , which ideally covers the test vessel with a metal mirror (see Tollens sample ).
Individual evidence
- ↑ a b Data sheet D - (+) - Altrose at Acros, accessed on February 19, 2010.
- ↑ Data sheet L - (-) - Altrose at Acros, accessed on February 19, 2010.
- ↑ a b Data sheet D-Altrose, ≥97.0% (HPLC) from Sigma-Aldrich , accessed on February 5, 2013 ( PDF ).
- ↑ a b Data sheet L-Altrose, ≥97.0% (HPLC) from Sigma-Aldrich , accessed on February 5, 2013 ( PDF ).
- ↑ Jürg Hunziker: Carbohydrate Chemistry ( Memento from May 10, 2008 in the Internet Archive ), April 1, 2007.