Galactose
| Structural formula | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
||||||||||||||||
| Fischer projection , open-chain representation | ||||||||||||||||
| General | ||||||||||||||||
| Surname |
|
|||||||||||||||
| other names |
|
|||||||||||||||
| Molecular formula | C 6 H 12 O 6 | |||||||||||||||
| Brief description |
white odorless solid |
|||||||||||||||
| External identifiers / databases | ||||||||||||||||
|
||||||||||||||||
| properties | ||||||||||||||||
| Molar mass | 180.16 g mol −1 | |||||||||||||||
| Physical state |
firmly |
|||||||||||||||
| density |
1.5 g cm −3 ( D- shape) |
|||||||||||||||
| Melting point |
|
|||||||||||||||
| solubility |
|
|||||||||||||||
| safety instructions | ||||||||||||||||
|
||||||||||||||||
| As far as possible and customary, SI units are used. Unless otherwise noted, the data given apply to standard conditions . | ||||||||||||||||
The galactose (technical language case), shortly Gal , also galactose (traditional spelling of ancient Greek γάλα Gála , genitive: τοῦ γάλακτος Tou Galaktos , German "milk") or mucus Sugar is a naturally occurring chemical compound from the group of monosaccharides ( simple sugars ). Galactose comes e.g. B. in most living things as a component of oligo- and polycondensates of carbohydrates in various mucous membranes , from which the German name is derived. Compared to sucrose , a 10% D- galactose solution has a sweetening power of 63%.
properties
Galactose is a hexose and like all hexoses has the empirical formula C 6 H 12 O 6 . It is stereoisomeric (more precisely a C4 epimer ) to glucose and belongs to the subgroup of aldohexoses .
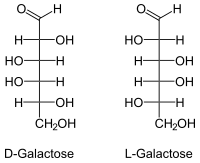
Galactose, like most natural sugars, has the D configuration ; L- galactose is only of secondary importance in practice. If "galactose" is mentioned without any additional name ( prefix ), then D- galactose is always meant.
| D -Galactose - spellings | ||
|---|---|---|
| Wedge formula | Haworth notation | |

|
 α- D- galactofuranose |
 β- D- galactofuranose |
 α- D- galactopyranose |
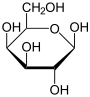 β- D- galactopyranose |
|
Each ring shape and each stereoisomer has its own CAS number:
| open- chain |
Furanosis | Pyranosis | |
|---|---|---|---|
| D- galactose | 59-23-4 | 19217-07-3 | 10257-28-0 |
| L -galactose | 15572-79-9 | 41846-90-6 | 39392-65-9 |
Behavior in aqueous solution
In aqueous solution, an intramolecular ring closure sometimes occurs, so that an equilibrium is established between the aldoform and the two ring forms ( furanose form and pyranose form):
At 20 ° C, D- galactose dissolved in water is 32% in the α-pyranose form, 64% in the β-pyranose form, 1% in the α-furanose form and 3% in the β-furanose form.
Specific rotation values
- α- D -Galactopyranose (= six-membered ring): [α] 20 D = + 150.7 °
- β- D- galactopyranose: [α] 20 ° / D = + 52.8 °
Galactose shows mutarotation . Rotation value of the aqueous solution: [α] 20 ° / D = + 80.2 °
Energy metabolism
Galactose is made available for glycolysis in a multi-step process through epimerization . The following reaction steps are carried out:
- With the help of galactokinase (GK, EC 2.7.1.6 ), galactose ( 1 ) is phosphorylated to galactose-1-phosphate ( 2 ) with consumption of ATP .
- In the next step, the enzyme galactose-1-phosphate uridyltransferase (GALT, EC 2.7.7.12 ) converts the educt with the participation of UDP-glucose ( 3 ): UDP-galactose ( 4 ) and glucose-1-phosphate ( 5 ). This enzyme is defective in galactosemia .
- The enzyme phosphoglucomutase (PGM, EC 5.4.2.2 ) can isomerize glucose-1-phosphate to glucose-6-phosphate ( 6 ), an intermediate in glycolysis. In addition, glucose-1-phosphate together with UTP (uridine triphosphate) can be regenerated to UDP-glucose by UDP-glucose pyrophosphorylase ( EC 2.7.7.9 ).
- The enzyme UDP-glucose-4-epimerase (UGE, EC 5.1.3.2 ) ensures that UDP-glucose can be regenerated from UDP-galactose. It can be used again for the reaction in the second step or for glycogen biosynthesis.
Occurrence
In addition to being a monosaccharide, galactose also occurs as a building block in di- (e.g. lactose ), oligo- (e.g. raffinose ) and polysaccharides (e.g. agarose ). It is also a component of proteoglycans and glycolipids . With UDP-galactose, the organism provides sufficient raw material for this, even with galactose-free food.
In the lactating mammary gland , lactose from UDP-galactose and glucose is made available as an important energy source for infants with the help of lactose synthetase in breast milk . The lactose is split into glucose and galactose in the small intestine by the enzyme lactase and fed into the energy metabolism.
use
Galactose is used as a dietary supplement or sugar substitute.
Galactose as "brain sugar"
Galactose serves as an insulin-independent energy source for the brain and thus supports the ability to concentrate as well as memory. This is particularly relevant for patients with neurodegenerative diseases, as they often show insulin resistance. Studies in rats show the positive effects of galactose for the treatment of cognitive deficits and the potential of galactose in the treatment of neurodegenerative diseases.
Galactose and diabetes
Because of the insulin-independent cellular uptake of galactose, it has little effect on blood sugar levels. The glycemic index of galactose is 20 (glucose = 100). As early as the 1930s, doctors at the Berlin Charité were successfully treating diabetes patients with galactose as a sugar substitute.
Galactose in sports
Galactose is also used in the body for the production of glycoproteins and for the detoxification of ammonia. Therefore, galactose is used as a nutritional supplement in sports during or after training. Ammonia is continuously formed during training, which is associated with a drop in performance. By taking galactose, the toxin is transported out of the cell faster and the muscle remains more efficient and can also recover better.
illness
A hereditary disease in which the affected person cannot use galactose at all due to an enzyme defect is called galactosemia . It comes into play immediately after birth.
Studies show that chronic overdose of D- galactose can aggravate brain aging in mice through increased inflammation and oxidative stress.
Web links
Individual evidence
- ↑ a b c d e Data sheet D - (+) - Galactose, 98% from AlfaAesar, accessed on December 26, 2019 ( PDF )(JavaScript required) .
- ↑ a b c Data sheet L - (-) - Galactose, 98% from AlfaAesar, accessed on December 26, 2019 ( PDF )(JavaScript required) .
- ↑ a b c Entry on galactose. In: Römpp Online . Georg Thieme Verlag, accessed on June 1, 2014.
- ^ Hans-Dieter Belitz , Werner Grosch, Peter Schieberle : Textbook of food chemistry. 6th, completely revised edition. Springer, Berlin a. a. 2008, ISBN 978-3-540-73201-3 , p. 263.
- ↑ Jürg Hunziker: Carbohydrate Chemistry ( Memento from May 10, 2008 in the Internet Archive ), April 1, 2007.
- ^ Hans-Dieter Belitz, Werner Grosch, Peter Schieberle: Textbook of food chemistry. 6th, completely revised edition. Springer, Berlin a. a. 2008, ISBN 978-3-540-73201-3 , p. 269.
- ↑ Kelly T. Dineley, Jordan B. Jahrling, Larry Denner: Insulin resistance in Alzheimer's disease . In: Neurobiology of Disease . tape 72 , p. 92-103 , doi : 10.1016 / j.nbd.2014.09.001 .
- ↑ Lina Ma, Jieyu Wang, Yun Li: Insulin resistance and cognitive dysfunction . In: Clinica Chimica Acta . tape 444 , p. 18-23 , doi : 10.1016 / j.cca.2015.01.027 .
- ↑ Melita Salkovic-Petrisic, Jelena Osmanovic-Barilar, Ana Knezovic, Siegfried Hoyer, Kurt Mosetter: Long-term oral galactose treatment prevents cognitive deficits in male Wistar rats treated intracerebroventricularly with streptozotocin . In: Neuropharmacology . tape 77 , p. 68-80 , doi : 10.1016 / j.neuropharm.2013.09.002 .
- ↑ H. Kosterlitz, HW Wedler: Investigations into the utilization of galactose in physiological and pathological states . In: Journal for all of experimental medicine . tape 87 , no. 1 , December 1, 1933, p. 397-404 , doi : 10.1007 / BF02610497 .
- ↑ Martin Roser, Djuro Josic, Maria Kontou, Kurt Mosetter, Peter Maurer: Metabolism of galactose in the brain and liver of rats and its conversion into glutamate and other amino acids . In: Journal of Neural Transmission . tape 116 , no. 2 , February 1, 2009, p. 131 , doi : 10.1007 / s00702-008-0166-9 .
- ↑ Thazin Shwe, Wasana Pratchayasakul, Nipon Chattipakorn, Siriporn C. Chattipakorn: Role of D-galactose-induced brain aging and its potential used for therapeutic interventions . In: Experimental Gerontology . tape 101 , November 10, 2017, p. 13-36 , doi : 10.1016 / j.exger.2017.10.029 .
