Lactase
| Lactase | ||
|---|---|---|
| Properties of human protein | ||
| Mass / length primary structure | 1061 amino acids; 160 kDa | |
| Secondary to quaternary structure | single pass type 1 membrane protein, dimer | |
| Precursor | Pre-pro-LPH (1927 amino acids; 219 kDa) | |
| Identifier | ||
| Gene names | LCT ; LAC; LPH; LPH1 | |
| External IDs |
|
|
| Drug information | ||
| ATC code | A09 AA04 | |
| Enzyme Classifications | ||
| EC, category | 3.2.1.108 , glycosidase | |
| Response type | hydrolysis | |
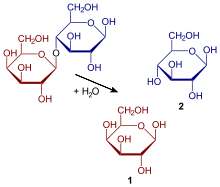
| Substrate | Lactose + H 2 O | |
| Products | D-galactose + D-glucose | |
| EC, category | 3.2.1.62 , glycosidase | |
| Response type | hydrolysis | |
| Substrate | Glycosyl-N-acylsphingosine + H 2 O | |
| Products | N-acylsphingosine + sugar | |
| Occurrence | ||
| Parent taxon | Mammals | |
Lactase or lactase ( gene name LCT ; also Lactase- Phlorizin - Hydrolase , abbreviation LPH ) is the name of an enzyme found in the intestine that splits lactose (milk sugar) into its components galactose (mucous sugar) and glucose (grape sugar). Without this chemical reaction, the constituents of lactose cannot be absorbed through the mucous membrane of the small intestine. In humans, the enzyme is normally produced in the small intestine in childhood , and in Europe later in adulthood in most people. Lactase deficiency can have several causes (see Lactose intolerance ) and leads to digestive problems in two thirds of those affected when lactose is consumed.
The food industry uses enzymes that can also hydrolyze lactose and are classified under the generic term β-galactosidases . In scientific parlance, lactase should only be used for mammalian β-galactosidases. Since the production of human lactase would be uneconomical, only microbial β-galactosidases from safe and approved bacteria ( Escherichia coli K-12), yeasts ( Kluyveromyces lactis ) or filamentous fungi are produced biotechnologically for food technology. The hydrolysis of lactose leads to the reduction of milk sugar, whereby glucose and galactose are formed, which contribute to an increased sweetness in lactose-free milk. Lactose-free foods are not only sold to lactose-intolerant people, lactose-free ice cream, for example, has more favorable processing properties and a finer texture due to other crystallization properties.
In addition to lactase activity, the enzyme has another glycosylceramidase activity, which is why it is referred to in the specialist literature as lactase phlorizin hydrolase . Structurally, the LCT gene consists of four similar domains, with lactase activity taking place on the fourth and phlorizin hydrolase on the third domain.
As an integral membrane protein , LPH is localized in the brush border membrane of the columnar main cells of the villi epithelium of the small intestine of all mammals. Mutations in the LCT gene can be the cause of hereditary variants of lactose intolerance via a reduction in LPH activity . On the other hand, certain other mutations lead to lactase persistence , especially in residents of the northern hemisphere , via an increase in LPH production or its retention in adulthood . However, the normal condition is a decrease in LPH production after breastfeeding .
evolution
Due to the fourfold glycosidase-1 domain, two gene doublings in the evolution of the LCT gene can be assumed. That the last of these doublings took place before the development of vertebrates can be seen from the fact that the genes orthologous to LCT already have four glycosidase-1 domains in several fish species.
Its selection in milk-drinking cultures is responsible for the evolution of the lactase persistence mutation . The coevolution propagated by Beja-Pereira and others with a similar mutation in dairy cows is now the most likely explanation for the high proportion of persistence in the human population and its distribution profile.
biosynthesis
The LPH for encoding gene LCT is on the second chromosome (2q21). The LCT gene spans 17 exons and 49,340 base pairs. After transcription , mRNA is produced which contains 6274 bases and is translated into a 1927 amino acid long molecule pre-pro-LPH . This contains four similar glycosidase 1 domains and a signal sequence that causes prepro-LPH to be transported from the ER to the cell membrane . After removing the signal sequence (19 amino acids), peptidases cut the molecule into two parts: LPHβ (1061 amino acids) and another protein LPHα (847 amino acids), which is probably necessary for the transport of the LPHβ dimer to the membrane, which is also phosphorylated and finally is glycosylated .
Catalyzed reaction
Lactose is split into glucose and galactose . The optimal conditions for lactase are at a pH value of six, at a temperature of 25 ° C.
Industrial use
Commercially produced lactase is obtained from yeasts such as Kluyveromyces fragilis . The enzyme obtained in this way is offered in the form of tablets and capsules so that people with lactose intolerance can consume dairy products. There is also lactose-free milk in which the lactose has already been broken down by adding lactase; This means that this milk is edible for people who lack the enzyme lactase.
Lactase is used in the production of ice cream because the breakdown products glucose and galactose are sweeter than lactose and therefore the addition of sugar can be restricted. In addition, lactose crystallizes at temperatures such as those in ice cream, while the breakdown products are still present in dissolved form and thus give the ice cream a finer texture.
Quantities are expressed in FCC units. 1000 FCC units can break down 5 grams of milk sugar under optimal conditions. Among other things, it depends on the viscosity of the solution. A larger amount of the enzyme is required in thick solutions. Since milk flocculates in the acidic environment of the stomach, a larger amount of lactase is also required in order to break down the lactose completely if lactase is consumed directly when consuming dairy products.
Web links
Individual evidence
- ↑ Orthologist at OMA
- ^ Carl A. Batt, Mary Lou Tortorello: Encyclopedia of Food Microbiology . 2nd Edition. Elsevier, 2014. ISBN 978-0-12-384730-0 .
- ↑ UniProt entry
- ↑ P. Born: DD of unspecific abdominal complaints: the carbohydrate malabsorption . In: Münch. Med. Wschr. . 139, No. 29, 1997, pp. 32 / 436-36 / 440.
- ↑ JC Arribas, AG Herrero u. a .: Differential mechanism-based labeling and unequivocal activity assignment of the two active sites of intestinal lactase / phlorizin hydrolase. In: European Journal of Biochemistry Volume 267, Number 24, December 2000, pp. 6996-7005, PMID 11106409 .
-
↑ Lactase of the green globefish in Ensembl
Lactase of the Medaka - ↑ A. Beja-Pereira, G. Luikart u. a .: Gene-culture coevolution between cattle milk protein genes and human lactase genes. In: Nature genetics . Volume 35, Number 4, December 2003, pp. 311-313, doi : 10.1038 / ng1263 . PMID 14634648 . (Review).
- ↑ GeneID 3938
- ↑ ENSEMBL entry
- ↑ N. Mantei, M. Villa u. a .: Complete primary structure of human and rabbit lactase-phlorizin hydrolase: implications for biosynthesis, membrane anchoring and evolution of the enzyme. In: The EMBO journal . Volume 7, Number 9, September 1988, pp. 2705-2713. PMID 2460343 . PMC 457059 (free full text).
- ^ HY Naim, R. Jacob et al. a .: The pro region of human intestinal lactase-phlorizin hydrolase. In: The Journal of biological chemistry . Volume 269, Number 43, October 1994, pp. 26933-26943, PMID 7523415 .
- ↑ Skovbjerg H, Sjöström H, Norén O: Purification and characterization of amphiphilic lactase / phlorizin hydrolase from human small intestine . In: Eur. J. Biochem. . 114, No. 3, March 1981, pp. 653-661. doi : 10.1111 / j.1432-1033.1981.tb05193.x . PMID 6786877 .
- ↑ Hermida C, Corrales G, Cañada FJ, Aragón JJ, Fernández-Mayoralas A: Optimizing the enzymatic synthesis of beta-D-galactopyranosyl-D-xyloses for their use in the evaluation of lactase activity in vivo . In: Bioorganic & Medicinal Chemistry . 15, No. 14, July 2007, pp. 4836-4840. doi : 10.1016 / j.bmc.2007.04.067 . PMID 17512743 .
- ↑ J. Beuth: Good through cancer therapy. Thieme, 2011, ISBN 978-3-8304-6399-3 . (on-line)